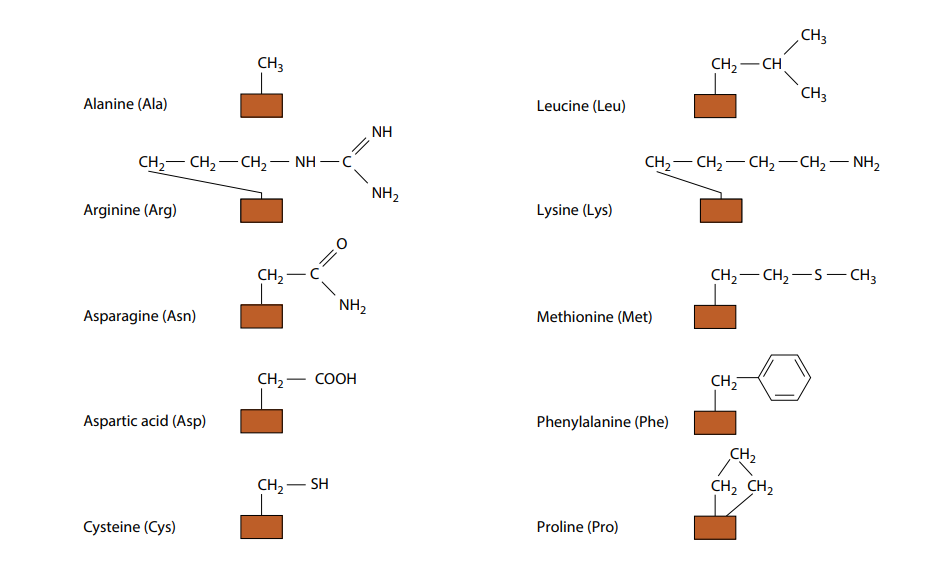
Amino Acids That Can Form Hydrogen Bonds
Amino Acids That Can Form Hydrogen Bonds - Web can amino form hydrogen bonds? Conditional amino acids include arginine, cysteine, glutamine, glycine, proline, and tyrosine. Hydrophilic amino acids have oxygen and nitrogen atoms, which can form hydrogen bonds with water. The α helix is stabilized by hydrogen bonds between an amide hydrogen of one amino acid and a carbonyl oxygen four amino acids away. Web the polar, uncharged amino acids serine (ser, s), threonine (thr, t), asparagine (asn, n) and glutamine (gln, q) readily form hydrogen bonds with water and other amino acids. • 2 comments ( 13 votes) flag laurent 8 years ago Hydrophobic side chains interact with each other via weak van der waals interactions. For example, the amino acid serine contains an. This is a classic situation where hydrogen bonding can occur. Hydrogen bonding and ionic bonding (figure 1).
Conditional amino acids include arginine, cysteine, glutamine, glycine, proline, and tyrosine. Web 1 day agoand inside is where the amino acids link up to form a protein. Web hydrogen bonding between amino acids in a linear protein molecule determines the way it folds up into its functional configuration. As a result, why does 'hydrogen bonding' occur to form secondary structures such as alpha helices and beta pleated sheets, rather than 'ionic bonding'? The pocket allows the amino acids to be positioned in exactly the right place so that a peptide bond can be made, says yonath. Web can amino form hydrogen bonds? Hydrogen bonding and ionic bonding (figure 1). Web lots of amino acids contain groups in the side chains which have a hydrogen atom attached to either an oxygen or a nitrogen atom. Hydrophobic side chains interact with each other via weak van der waals interactions. Their solubility depends on the size and nature of the side chain.
Web how amino acids form peptide bonds (peptide linkages) through a condensation reaction (dehydration synthesis). Example of salt bridge between amino acids glutamic acid and lysine demonstrating electrostatic interaction and hydrogen bonding. These form hydrogen bonds to a purine, pyrimidine, or phosphate group in dna. These atoms have an unequal distribution of electrons, creating a polar molecule that can interact and form hydrogen bonds with water. Web the essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. They do not ionize in normal conditions, though a prominent exception being the catalytic serine in serine proteases. As a result, why does 'hydrogen bonding' occur to form secondary structures such as alpha helices and beta pleated sheets, rather than 'ionic bonding'? Web two amino acids, serine and threonine, contain aliphatic hydroxyl groups (that is, an oxygen atom bonded to a hydrogen atom, represented as ―oh). Arginine, histidine, lysine, serine, threonine, asparagine, glutamine, tryptophan and tyrosine. The remaining amino acids have substituents that carry either negative or positive charges in aqueous solution at neutral ph and are therefore strongly hydrophilic.
organic chemistry Which atoms in a given amino acid are able to form
Ion pairing is one of the most important noncovalent forces in chemistry, in. Web of the 20 common amino acids, those with side groups capable of hydrogen bond formation are: Top voted questions tips & thanks gio 8 years ago sorry if this seems like an awfully basic question, but why does o get a negative charge at 4:01 ?.
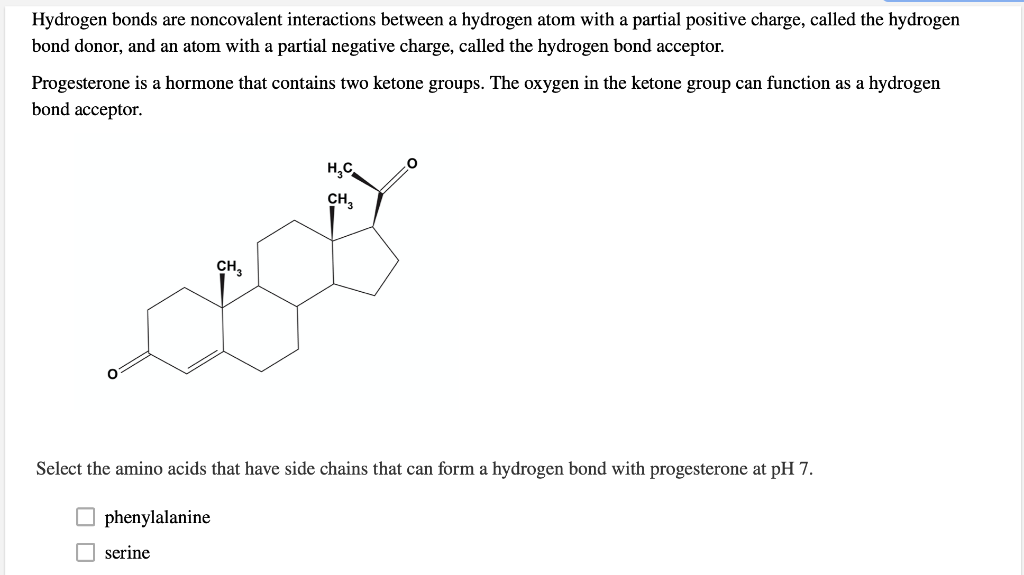
Solved Select the amino acids that have side chains that can
Web an important feature of the structure of proteins (which are polypeptides, or polymers formed from amino acids) is the existence of the peptide link, the group ―co―nh―, which appears between each pair of adjacent amino acids. The α helix is stabilized by hydrogen bonds between an amide hydrogen of one amino acid and a carbonyl oxygen four amino acids.
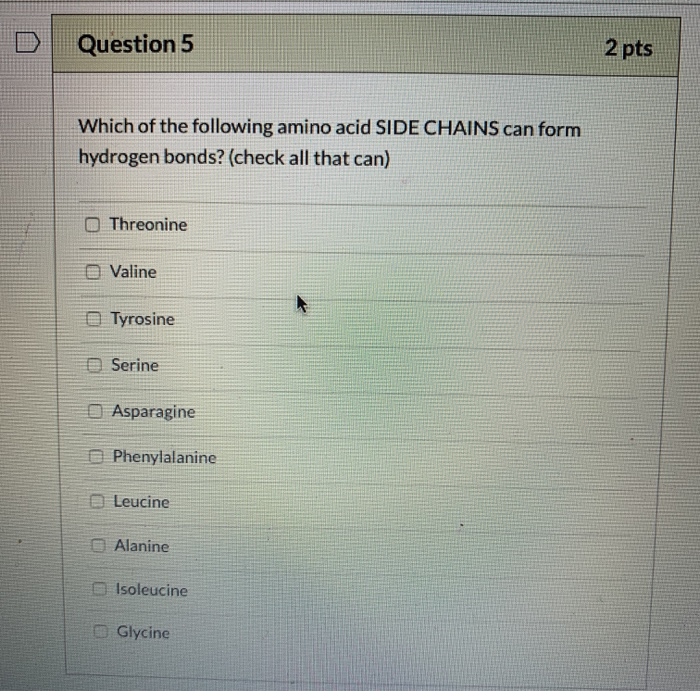
Solved Question 5 2 pts Which of the following amino acid
Web the hydrogen is covalently attached to one of the atoms (called the hydrogen bond donor) and interacts with the other (the hydrogen bond acceptor). This link provides an nh group that can form a hydrogen bond to a suitable acceptor atom and an oxygen atom, which. Arginine, histidine, lysine, serine, threonine, asparagine, glutamine, tryptophan and tyrosine. Hydrogen bonding and.
Amino Acids 20 Standard Amino Acids The Best Information
Web hydrogen bonding between amino acids in a linear protein molecule determines the way it folds up into its functional configuration. Their solubility depends on the size and nature of the side chain. Hydrophilic amino acids have oxygen and nitrogen atoms, which can form hydrogen bonds with water. Tyrosine possesses a hydroxyl group in the aromatic ring, making it a.
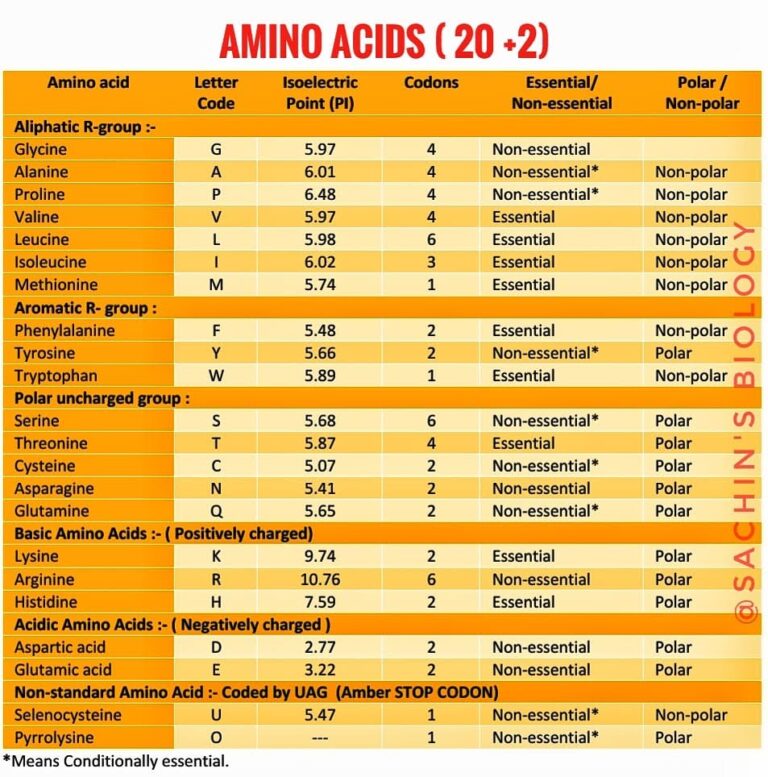
Amino Acid Side Chains Study Sheet
Web hydrogen bonding between amino acids in a linear protein molecule determines the way it folds up into its functional configuration. Web the essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. This link provides an nh group that can form a hydrogen bond to a suitable acceptor atom and an oxygen atom, which. This.
aqueoussolution L'acide glutamique et l'arginine peuventils former
Web an important feature of the structure of proteins (which are polypeptides, or polymers formed from amino acids) is the existence of the peptide link, the group ―co―nh―, which appears between each pair of adjacent amino acids. For example, the amino acid serine contains an. Web the essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and.
Two amino acids are joined together by
As a result, why does 'hydrogen bonding' occur to form secondary structures such as alpha helices and beta pleated sheets, rather than 'ionic bonding'? Web charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. These form hydrogen bonds to a purine, pyrimidine, or phosphate group in dna. These atoms have.
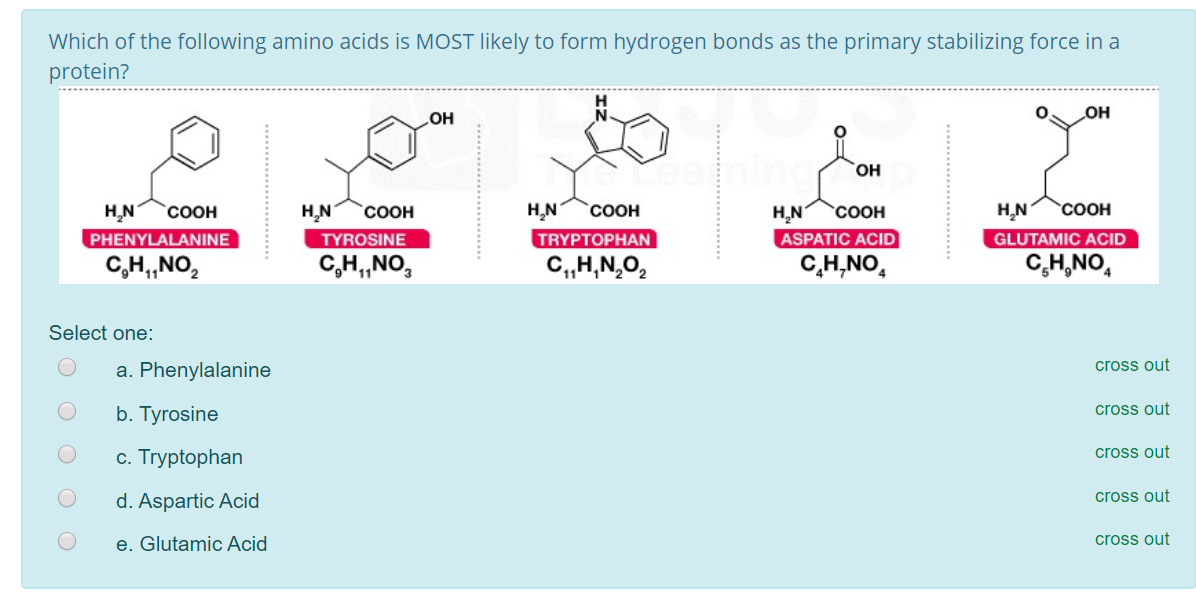
Solved Which of the following amino acids is MOST likely to
They do not ionize in normal conditions, though a prominent exception being the catalytic serine in serine proteases. Ion pairing is one of the most important noncovalent forces in chemistry, in. Top voted questions tips & thanks gio 8 years ago sorry if this seems like an awfully basic question, but why does o get a negative charge at 4:01.
Print USC Bridge 2.5 proteins flashcards Easy Notecards
Their other properties varying for each particular amino acid. They do not ionize in normal conditions, though a prominent exception being the catalytic serine in serine proteases. This is a classic situation where hydrogen bonding can occur. Web lots of amino acids contain groups in the side chains which have a hydrogen atom attached to either an oxygen or a.
Amino Acid and PeptidesAn Inevitable Organic Compounds Plantlet
Ion pairing is one of the most important noncovalent forces in chemistry, in. Their other properties varying for each particular amino acid. Web an important feature of the structure of proteins (which are polypeptides, or polymers formed from amino acids) is the existence of the peptide link, the group ―co―nh―, which appears between each pair of adjacent amino acids. Web.
This Is A Classic Situation Where Hydrogen Bonding Can Occur.
Tyrosine possesses a hydroxyl group in the aromatic ring, making it a phenol derivative. Web of the 20 common amino acids, those with side groups capable of hydrogen bond formation are: The 20 standard amino acids name structure (at neutral ph) nonpolar (hydrophobic) r For example, the amino acid serine contains an.
These Form Hydrogen Bonds To A Purine, Pyrimidine, Or Phosphate Group In Dna.
Web how amino acids form peptide bonds (peptide linkages) through a condensation reaction (dehydration synthesis). Top voted questions tips & thanks gio 8 years ago sorry if this seems like an awfully basic question, but why does o get a negative charge at 4:01 ? Hydrogen bonding and ionic bonding (figure 1). • 2 comments ( 13 votes) flag laurent 8 years ago
Web Charged Amino Acid Side Chains Can Form Ionic Bonds, And Polar Amino Acids Are Capable Of Forming Hydrogen Bonds.
Their other properties varying for each particular amino acid. As a result, why does 'hydrogen bonding' occur to form secondary structures such as alpha helices and beta pleated sheets, rather than 'ionic bonding'? Web amino acids are crystalline solids which usually are water soluble and only sparingly dissoluble in organic solvents. Web can amino form hydrogen bonds?
Web Hydrogen Bonds.is The Existence Of The Peptide Link, The Group ―Co―Nh―, Which Appears Between Each Pair Of Adjacent Amino Acids.
Web an important feature of the structure of proteins (which are polypeptides, or polymers formed from amino acids) is the existence of the peptide link, the group ―co―nh―, which appears between each pair of adjacent amino acids. Web lots of amino acids contain groups in the side chains which have a hydrogen atom attached to either an oxygen or a nitrogen atom. The pocket allows the amino acids to be positioned in exactly the right place so that a peptide bond can be made, says yonath. Hydrophobic side chains interact with each other via weak van der waals interactions.