Can Ammonia Form Hydrogen Bonds
Can Ammonia Form Hydrogen Bonds - Web actually, an ammonia molecule can form two hydrogen bonds, not one. In some ways it makes. 8.9k views 1 year ago. Web ammonium ion still has nitrogen bearing a partial negative charge. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. Web in ammonia (nh 3 ), the number of hydrogen bonds formed is limited since in each nitrogen there is only one lone pair of electrons which is shared with one hydrogen. In a group of ammonia molecules, there aren't enough. The lone pair on nitrogen can accept one hydrogen to form a hydrogen bond, and the three hydrogen. Web these three materials have four fundamental elements, o, n, c, and h, that are the building blocks of amino acids.
Web ammonia can form four hydrogen bonds per molecule. The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more. When the hydrogen bonds between water molecules are broken, they can be replaced by equivalent bonds. Due to the electronegativity difference. Web ammonia has the ability to form hydrogen bonds. In some ways it makes. The lone pair of electrons present in nitrogen is equal to one. Web these three materials have four fundamental elements, o, n, c, and h, that are the building blocks of amino acids. Web as expected, nh(3) is observed to be a nearly universal proton acceptor, accepting hydrogen bonds fr. Web paradoxically ammonia is a better carrier of hydrogen than hydrogen itself as its properties make transportation and storage straightforward.
Theoretical studies of the reaction. An important difference in terms of hydrogen bonding between ammonia, nh3 , and water,. When the hydrogen bonds between water molecules are broken, they can be replaced by equivalent bonds. Spectroscopic characterizations of the stereochemistry. Web as expected, nh(3) is observed to be a nearly universal proton acceptor, accepting hydrogen bonds fr. Web in ammonia (nh 3 ), the number of hydrogen bonds formed is limited since in each nitrogen there is only one lone pair of electrons which is shared with one hydrogen. Ammonia has electronegative atom nitrogen connected to hydrogen. Web and it has total three hydrogen bonding by the acceptance and donation. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. Due to the electronegativity difference.
What is Ammonia? nh3 fuels
In some ways it makes. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. Web in ammonia (nh 3 ), the number of hydrogen bonds formed is limited since in each nitrogen there is only one lone pair of electrons which is shared with one hydrogen. The lone pair on nitrogen can accept one hydrogen to form a.
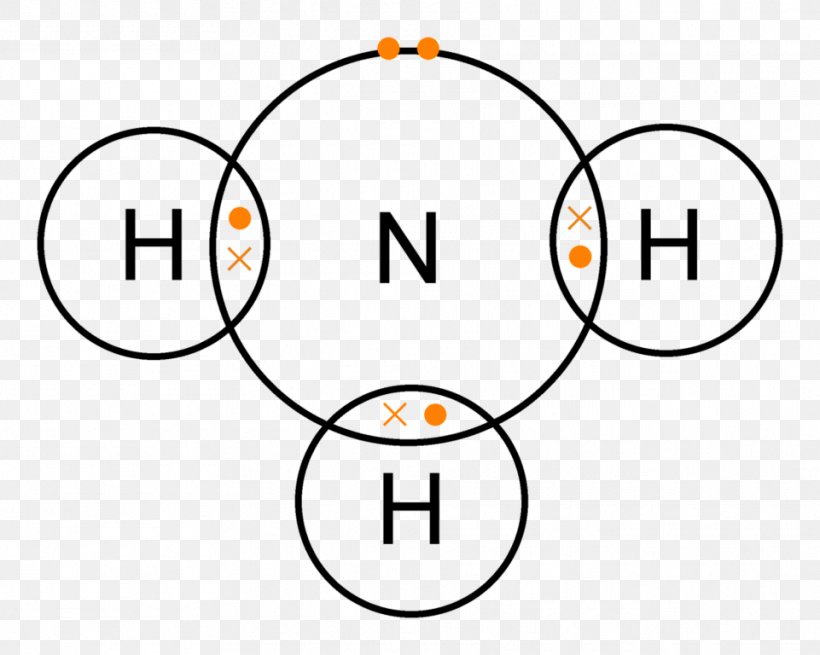
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG
Web answer (1 of 3): Web in the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair. Web actually, an ammonia molecule can form two hydrogen bonds, not one. However, we also know that. Web yes, nh3 forms hydrogen bonds.
Form I333 Anhydrous Ammonia Additive Credit printable pdf download
Web answer (1 of 3): An important difference in terms of hydrogen bonding between ammonia, nh3 , and water,. Web ammonia has the ability to form hydrogen bonds. Web paradoxically ammonia is a better carrier of hydrogen than hydrogen itself as its properties make transportation and storage straightforward. Web an ammonia molecule can donate and accept up to three hydrogen.
Properties of Water Presentation Biology
Liquid ammonia is constituted of ammonia molecules. Web these three materials have four fundamental elements, o, n, c, and h, that are the building blocks of amino acids. The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more. In some ways it makes. Web ammonia is.
Can Ammonia aka NH3 Be a Fuel?
Web an ammonia molecule can donate and accept up to three hydrogen bonds. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. 1 ammonia (nh 3) forms a weakly hydrogen. Liquid ammonia is constituted of ammonia molecules. However, we also know that.
Tokyo Tech Breaking Ammonia A New Catalyst to Generate Hydrogen from
Hydrogen bonding is the intermolecular forces acting between ammonia molecules. 8.9k views 1 year ago. The lone pair on nitrogen can accept one hydrogen to form a hydrogen bond, and the three hydrogen. Ammonia has electronegative atom nitrogen connected to hydrogen. In the gaseous state at high temperature, the ammonia molecules have sufficient kinetic energy to overcome the attractions to.
Ammonia Molecule Structure built with Atoms & Bonds from
Due to the electronegativity difference. However, we also know that. The lone pair of electrons present in nitrogen is equal to one. 8.9k views 1 year ago. Ammonia has electronegative atom nitrogen connected to hydrogen.
chemistry Intermolecular Hydrogen Bonding
Web yes, nh3 forms hydrogen bonds. In the gaseous state at high temperature, the ammonia molecules have sufficient kinetic energy to overcome the attractions to other ammonia molecules. The lone pair on nitrogen can accept one hydrogen to form a hydrogen bond, and the three hydrogen. Theoretical studies of the reaction. When the hydrogen bonds between water molecules are broken,.
Novel Technique Seamlessly Converts Ammonia to Green HydrogenUNIST News
Due to the electronegativity difference. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more. 1 ammonia (nh 3) forms a weakly hydrogen. Web in ammonia (nh 3 ),.
9 Hydrogen Bond Examples in Real Life StudiousGuy
In a group of ammonia molecules, there aren't enough. Web actually, an ammonia molecule can form two hydrogen bonds, not one. Web and it has total three hydrogen bonding by the acceptance and donation. The lone pair of electrons present in nitrogen is equal to one. Liquid ammonia is constituted of ammonia molecules.
Web These Three Materials Have Four Fundamental Elements, O, N, C, And H, That Are The Building Blocks Of Amino Acids.
Web an ammonia molecule can donate and accept up to three hydrogen bonds. However, we also know that. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. The lone pair of electrons present in nitrogen is equal to one.
In A Group Of Ammonia Molecules, There Aren't Enough.
To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. Web in ammonia (nh 3 ), the number of hydrogen bonds formed is limited since in each nitrogen there is only one lone pair of electrons which is shared with one hydrogen. Theoretical studies of the reaction. In some ways it makes.
Web Yes, Nh3 Forms Hydrogen Bonds.
Spectroscopic characterizations of the stereochemistry. Web and it has total three hydrogen bonding by the acceptance and donation. Web answer (1 of 3): In the gaseous state at high temperature, the ammonia molecules have sufficient kinetic energy to overcome the attractions to other ammonia molecules.
Web Actually, An Ammonia Molecule Can Form Two Hydrogen Bonds, Not One.
Web ammonia is an important molecule due to its wide use in the fertiliser industry. Of course it can interact with positively polarized hydrogens in an electrostatic way. Web ammonia has the ability to form hydrogen bonds. Web in the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair.

.PNG)