Can Nonpolar Molecules Form Hydrogen Bonds
Can Nonpolar Molecules Form Hydrogen Bonds - Identify types of intermolecular forces. It results from the attractive force between a. Web answer (1 of 8): Polymers that contain carbonyl or amide groups can form hydrogen bonds. 1 (a) conventional hydrogen bond compared with (b) a dihydrogen bond involving hydridic hydrogen atoms bonded to a strongly polarising element m. An example of a non polar molecule that is able to form hydrogen bonds is the amino acid tryptophan due its indole. Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions between nonpolar molecules). Web the molecule is symmetric. (i can't find a satisfying explanation) apparently, there's a misconception that says it's because of the. The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f.
Positively charged na ^+ + ions are surrounded by partial negative charges from the oxygen ends of the water molecules,. Web the molecule is symmetric. Such a bond is weaker than an ionic bond or. Identify types of intermolecular forces. An example of a non polar molecule that is able to form hydrogen bonds is the amino acid tryptophan due its indole. It results from the attractive force between a. Web the polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized. (i can't find a satisfying explanation) apparently, there's a misconception that says it's because of the. Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions between nonpolar molecules). The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f.
Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web the molecule is symmetric. The polarity of these molecules indicates that they will. It results from the attractive force between a. Propane is nonpolar, because it is symmetric, with h atoms. 1 (a) conventional hydrogen bond compared with (b) a dihydrogen bond involving hydridic hydrogen atoms bonded to a strongly polarising element m. Polymers that contain carbonyl or amide groups can form hydrogen bonds. (i can't find a satisfying explanation) apparently, there's a misconception that says it's because of the. Web some examples of polar molecules which can hydrogen bond are ammonia (#nh_3#) and methanol (#ch_3oh#). Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions between nonpolar molecules).
Ch4 Polar Or Nonpolar Covalent Bond PPT The Chemistry of Life
Most non polar molecules don't. Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions between nonpolar molecules). 1 (a) conventional hydrogen bond compared with (b) a dihydrogen bond involving hydridic hydrogen atoms bonded to a strongly polarising element m. (i can't find a satisfying explanation) apparently, there's a misconception that says it's because.
Ch4 Polar Or Nonpolar Atom Closest To Negative Side Is Carbon Dioxide
Covalent molecules made of only one type of atom, like. (i can't find a satisfying explanation) apparently, there's a misconception that says it's because of the. Web answer (1 of 8): When they form a bond by contributing one electron each and then. An example of a non polar molecule that is able to form hydrogen bonds is the amino.
Ch4 Polar Or Nonpolar Covalent Bond A CH4 B H2O C CF4 D CH3F Non
It results from the attractive force between a. Positively charged na ^+ + ions are surrounded by partial negative charges from the oxygen ends of the water molecules,. Web answer (1 of 3): As hydrogen molecule is made up of two hydrogen atoms having equal electronegativity. Such a bond is weaker than an ionic bond or.
Ch4 Polar Or Nonpolar Covalent Bond PPT The Chemistry of Life
Examples include urea and polyurethane and the natural polymer. The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f. Web answer (1 of 3): Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions between nonpolar molecules). Web distinguish between the following three types of.
التربويون الجدد الفرق بين الروابط الأيونية والتساهمية The difference
The polarity of these molecules indicates that they will. Most non polar molecules don't. (i can't find a satisfying explanation) apparently, there's a misconception that says it's because of the. 1 (a) conventional hydrogen bond compared with (b) a dihydrogen bond involving hydridic hydrogen atoms bonded to a strongly polarising element m. Web because nonpolar molecules share their charges evenly,.
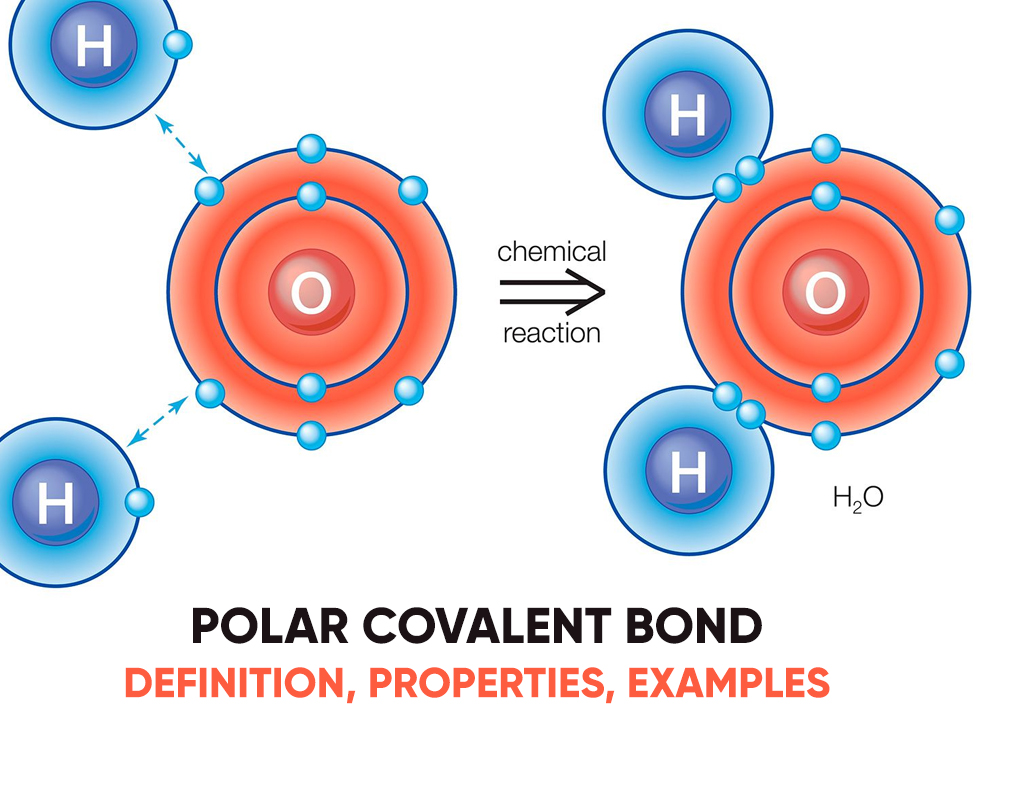
Polar Covalent Bond Definition, Properties, Examples
It results from the attractive force between a. If there is an acceptable difference. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web some examples of polar molecules which can hydrogen bond are ammonia (#nh_3#) and methanol (#ch_3oh#). Such a bond is weaker than an ionic bond.
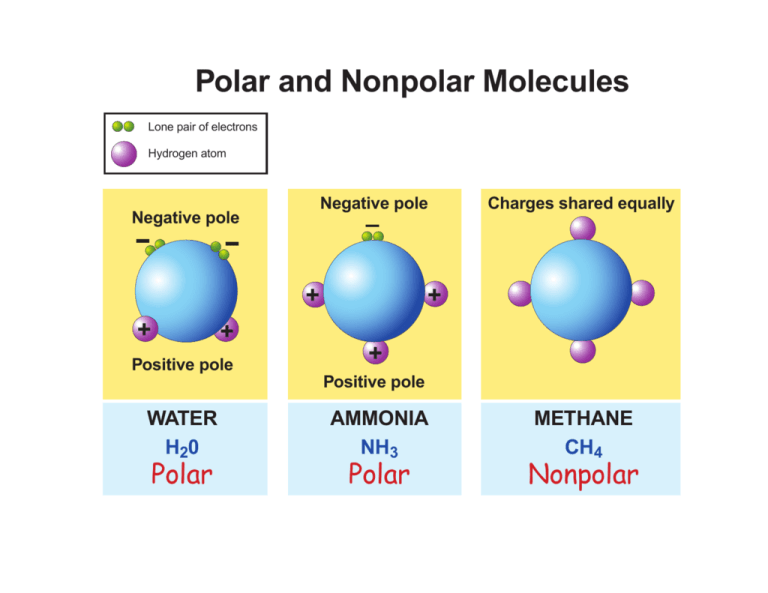
Polar and Nonpolar Molecules
Positively charged na ^+ + ions are surrounded by partial negative charges from the oxygen ends of the water molecules,. Covalent molecules made of only one type of atom, like. Web whether atoms form polar or nonpolar bonds depends on the difference between their electronegativity values. The polarity of these molecules indicates that they will. Propane is nonpolar, because it.
Chemical Bonds Anatomy and Physiology I
Polymers that contain carbonyl or amide groups can form hydrogen bonds. As hydrogen molecule is made up of two hydrogen atoms having equal electronegativity. Web water molecules form hydration shells around the ions: Web answer (1 of 8): The two oxygen atoms pull on the electrons by exactly the same amount.
Bonds That Hold Water Molecules Together / Intermolecular Forces
Polymers that contain carbonyl or amide groups can form hydrogen bonds. Identify types of intermolecular forces. Web the polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized. 1 (a) conventional hydrogen bond compared with (b) a dihydrogen bond involving hydridic hydrogen atoms bonded.
2.2A Covalent Bonds and Other Bonds and Interactions Medicine LibreTexts
Polymers that contain carbonyl or amide groups can form hydrogen bonds. Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions between nonpolar molecules). Positively charged na ^+ + ions are surrounded by partial negative charges from the oxygen ends of the water molecules,. Web the polarity of a covalent bond can be judged.
Most Non Polar Molecules Don't.
The polarity of these molecules indicates that they will. When they form a bond by contributing one electron each and then. Web distinguish between the following three types of intermolecular forces: 1 (a) conventional hydrogen bond compared with (b) a dihydrogen bond involving hydridic hydrogen atoms bonded to a strongly polarising element m.
It Results From The Attractive Force Between A.
The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f. Web water molecules form hydration shells around the ions: If there is an acceptable difference. (i can't find a satisfying explanation) apparently, there's a misconception that says it's because of the.
Web Answer (1 Of 8):
Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions between nonpolar molecules). Identify types of intermolecular forces. Web the polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized. Examples include urea and polyurethane and the natural polymer.
Such A Bond Is Weaker Than An Ionic Bond Or.
The two oxygen atoms pull on the electrons by exactly the same amount. Web answer (1 of 3): Polymers that contain carbonyl or amide groups can form hydrogen bonds. Web because nonpolar molecules share their charges evenly, they do not react to electrostatic charges like water does.