Chapter 2 Basic Chemistry Answer Key
Chapter 2 Basic Chemistry Answer Key - As a gas, essential to the. The starting materials consist of one green sphere and two purple spheres. Web chapter 2 basic chemistry. Chapter 2:atoms, and ions, and the periodic table. In this equation δg° is the gibbs free energy of a reaction under standard conditions of 1 atm (or 1 bar) for gases and 1 m for solutions. A molecule of oxygen, o 2, contains two oxygen atoms; Use the key choices to identify the substances described in the following statements. Can be transformed to the bonds of atp heat is: Chapter 2 basic chemistry 23 biochemistry: The chemical compositions of the body’s structures determine their function.
Web chapter 2 basic chemistry. The composition of living matter 14 use the key choices to identify the substances described in the following statements. Web 3 basic steps involved in enzyme action. Web chapter 2 basic chemistry. Web 1 / 37 flashcards learn test match created by alexis_denton5 terms in this set (37) the energy located in the bonds of food molecules: Can be transformed to the bonds of atp heat is: Insert the appropriate letter or corresponding term in the answer blanks. Web chapter 2 basic chemistry a&p worksheet learn with flashcards, games, and more — for free. Web test match created by adyson_baber terms in this set (74) the energy located in the bonds of food molecules can be transformed to the bond of atp and is a form of potential energy heat is thermal energy, infrared. This violates dalton’s postulate that that atoms are not created during a.
The composition of living matter 14. The subscript 2 in the formula must be used to distinguish the. Web this study guide is based off of biology by sylvia mader 10th edition. The chemical compositions of the body’s structures determine their function. This violates dalton’s postulate that that atoms are not created during a. Web study with quizlet and memorize flashcards containing terms like matter is defined as:, all matter is composed of various basic substances, like carbon or oxygen, these substances are called:, the six elements. Substances that ionize in water; This chapter presents the basics ofchemistry and biochemistry (the chemistry. The chapter is number 2 titled basic chemistry. Web chapter 2 basic chemistry.
Biologychapter2practicetest =LINK=
This chapter presents the basics ofchemistry and biochemistry (the chemistry. Substances that ionize in water; Insert the appropriate letter or corresponding term in the answer blanks. Chapter 2 basic chemistry 23 biochemistry: In this equation δg° is the gibbs free energy of a reaction under standard conditions of 1 atm (or 1 bar) for gases and 1 m for solutions.
CHAPTER 2 BASIC CHEMISTRY
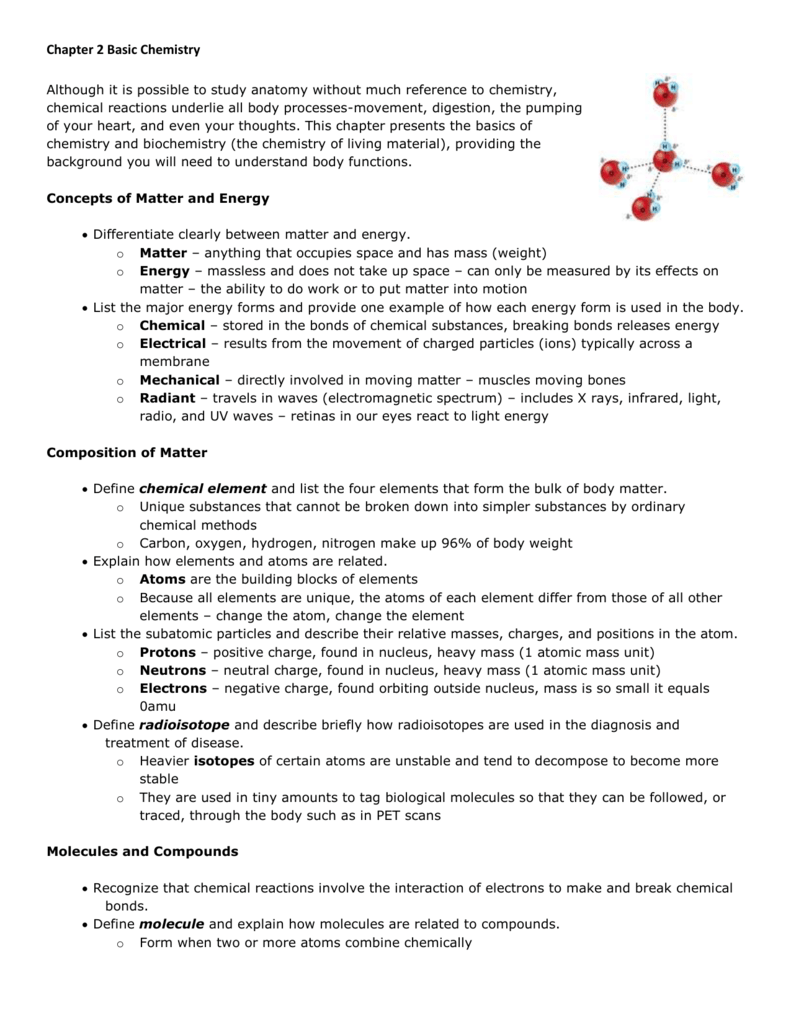
A molecule of oxygen, o 2, contains two oxygen atoms; In this equation δg° is the gibbs free energy of a reaction under standard conditions of 1 atm (or 1 bar) for gases and 1 m for solutions. Web details table of contents 1.0 introduction to chemistry 2.0 matter and change 3.0 measurement 4.0 atomic structure 5.0 electrons in atoms.
Gseb 12th Science Chemistry 09/03/2019 Paper Answer Key Gujarati Medium
As a gas, essential to the. The chapter is number 2 titled basic chemistry. Substances that ionize in water; Web writing lab reports and papers. Web this study guide is based off of biology by sylvia mader 10th edition.
Anatomy And Physiology Chapter 2 Basic Chemistry Answers Anatomical
The starting materials consist of one green sphere and two purple spheres. Web the chapter 2 basic chemistry answer key serves as an invaluable companion, providing students with immediate feedback, reinforcing key concepts, and enhancing their problem. This violates dalton’s postulate that that atoms are not created during a. Web what is an atomic number? The chemical compositions of the.
Chemistry Review 2 answer key
This chapter presents the basics ofchemistry and biochemistry (the chemistry. Can be transformed to the bonds of atp heat is: In this equation δg° is the gibbs free energy of a reaction under standard conditions of 1 atm (or 1 bar) for gases and 1 m for solutions. The products consist of two green spheres and two purple spheres. The.
Composition Of Matter Worksheet Answers Worksheet List
Chapter 2 basic chemistry 23 biochemistry: Webstep 2 of 2 (b) the substance whose composition and properties remains same, irrespective of wherever it is found is defined as chemical. The chapter is number 2 titled basic chemistry. Web chapter 2 basic chemistry. Web details table of contents 1.0 introduction to chemistry 2.0 matter and change 3.0 measurement 4.0 atomic structure.
Chapter 2 Chemical Basis Of Life Study Guide Answers Study Poster
The chemical compositions of the body’s structures determine their function. Web writing lab reports and papers. Insert the appropriate letter(s) or corresponding term(s) in the answer. Can be transformed to the bonds of atp heat is: In the learn based tool, prompt with term and copy the whole answer and paste it into the.
Chemistry Unit 5 Test Answer Key Chemistry Unit 1 Worksheet 6 Answer
Use the key choices to identify the substances described in the following statements. Web writing lab reports and papers. Chapter 2:atoms, and ions, and the periodic table. The products consist of two green spheres and two purple spheres. Web chapter 2 basic chemistry a&p worksheet learn with flashcards, games, and more — for free.
Anatomy And Physiology Chapter 2 Basic Chemistry Answers Anatomical
A molecule of oxygen, o 2, contains two oxygen atoms; Web the symbol for the element oxygen, o, represents both the element and one atom of oxygen. Insert the appropriate letter or corresponding term in the answer blanks. Can be transformed to the bonds of atp heat is: As a gas, essential to the.
chem study guide answer key Solution Chemical Reactions
Web details table of contents 1.0 introduction to chemistry 2.0 matter and change 3.0 measurement 4.0 atomic structure 5.0 electrons in atoms 6.0 the periodic table 7.0 chemical nomenclature. This violates dalton’s postulate that that atoms are not created during a. Chapter 2:atoms, and ions, and the periodic table. Web you’ll need to know this as you study chemistry ii..
Chapter 2:Atoms, And Ions, And The Periodic Table.
Chapter 2 basic chemistry 23 biochemistry: Can be transformed to the bonds of atp heat is: The chapter is number 2 titled basic chemistry. Web chapter 2 basic chemistry a&p worksheet learn with flashcards, games, and more — for free.
The Starting Materials Consist Of One Green Sphere And Two Purple Spheres.
Insert the appropriate letter or corresponding term in the answer blanks. Web what is an atomic number? Use the key choices to identify the substances described in the following statements. Webstep 2 of 2 (b) the substance whose composition and properties remains same, irrespective of wherever it is found is defined as chemical.
This Chapter Presents The Basics Ofchemistry And Biochemistry (The Chemistry.
The composition of living matter 14. This violates dalton’s postulate that that atoms are not created during a. Substances that ionize in water; Web details table of contents 1.0 introduction to chemistry 2.0 matter and change 3.0 measurement 4.0 atomic structure 5.0 electrons in atoms 6.0 the periodic table 7.0 chemical nomenclature.
As A Gas, Essential To The.
For example salt (sodium chloride) it is. Web 3 basic steps involved in enzyme action. In this equation δg° is the gibbs free energy of a reaction under standard conditions of 1 atm (or 1 bar) for gases and 1 m for solutions. Web chapter 2 basic chemistry.