Chapter 6 Study Guide Chemistry
Chapter 6 Study Guide Chemistry - Web chapter 6 emphasizes skills that are necessary for drawing mechanisms, while chapter 12 prepares the student for proposing syntheses. The idea of arranging the elements in a table according to their chemical and physical properties is attributed to ________. A system that can exchange mass and energy (usually in the form of heat) with its surroundings. In addition, each chapter contains numerous skillbuilders, each of which is. Unit 11 states of matter and intermolecular forces. Explain the difference between dissolving and reacting. Solid gas liquid all of these, the temperature at. Web study with quizlet and memorize flashcards containing terms like chemical bond, 3 types of chemical bonds, why do. Web our interactive player makes it easy to find solutions to chemistry: The structure of atoms, discrete molecules, complex network solids, and metals;
Web study with quizlet and memorize flashcards containing terms like which state of matter is characterized by having molecules close together, but moving randomly? Web study guide chapter 6 section 2 chemical reactions. Web study with quizlet and memorize flashcards containing terms like chemical bond, 3 types of chemical bonds, why do. Dissolving is a physical change and reacting is a. Unit 10 gases and kinetic molecular theory. Web our interactive player makes it easy to find solutions to chemistry: State the principle that explains why there must be the same number of atoms of each elements on each side of an equation. Explain the difference between dissolving and reacting. The above figure represents an open system. The structure of atoms, discrete molecules, complex network solids, and metals;
The idea of arranging the elements in a table according to their chemical and physical properties is attributed to ________. Web chapter 6 emphasizes skills that are necessary for drawing mechanisms, while chapter 12 prepares the student for proposing syntheses. Web chemistry chapter 6 study guide. Web livingston public schools / lps homepage Electron energy level neutron nucleus proton in your. Web our interactive player makes it easy to find solutions to chemistry: Unit 10 gases and kinetic molecular theory. Click the card to flip 👆. A substance that forms in a chemical reaction. The above figure represents an open system.
Century 21 Accounting Chapter 6 Study Guide Answers Study Poster
Explain the difference between dissolving and reacting. Dissolving is a physical change and reacting is a. We have covered quite a number of topics up to this point: B) read the following sections from chapter 7 and do the problems in those sections: Electron energy level neutron nucleus proton in your.
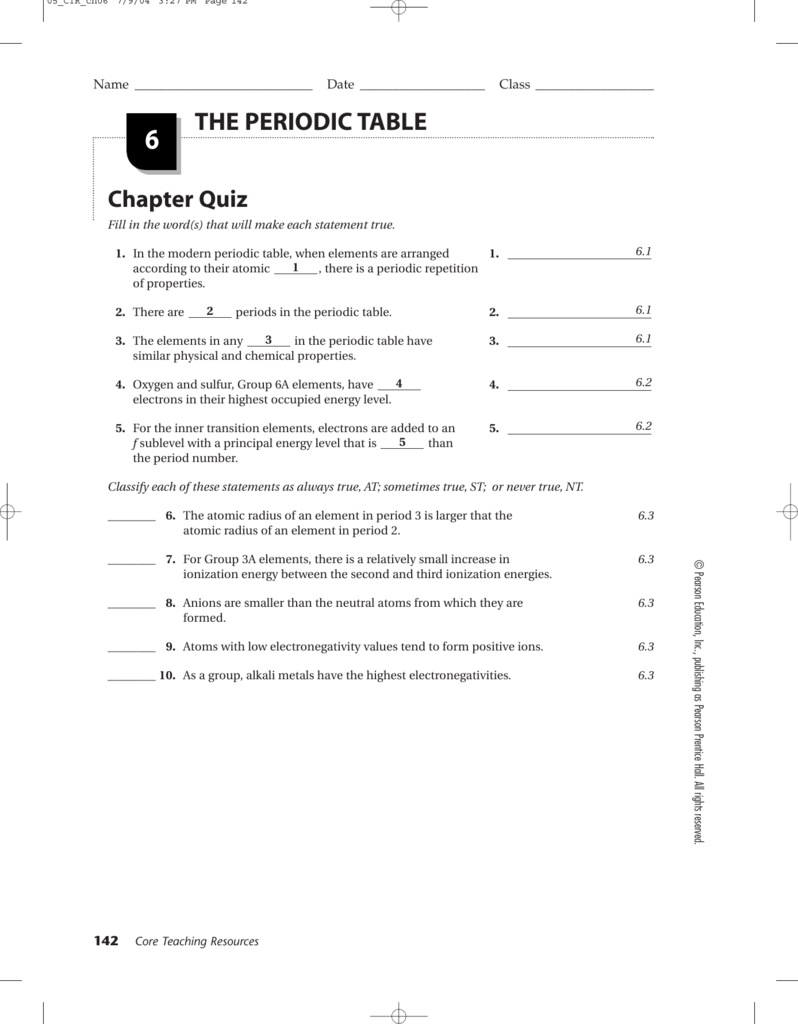
Chemistry Chapter 6 The Periodic Table Worksheet Answers
Web chemistry chapter 6 study guide. Dissolving is a physical change and reacting is a. The above figure represents an open system. Web michigan state university and uc bolder. Web unit 6 more about chemical reactions.
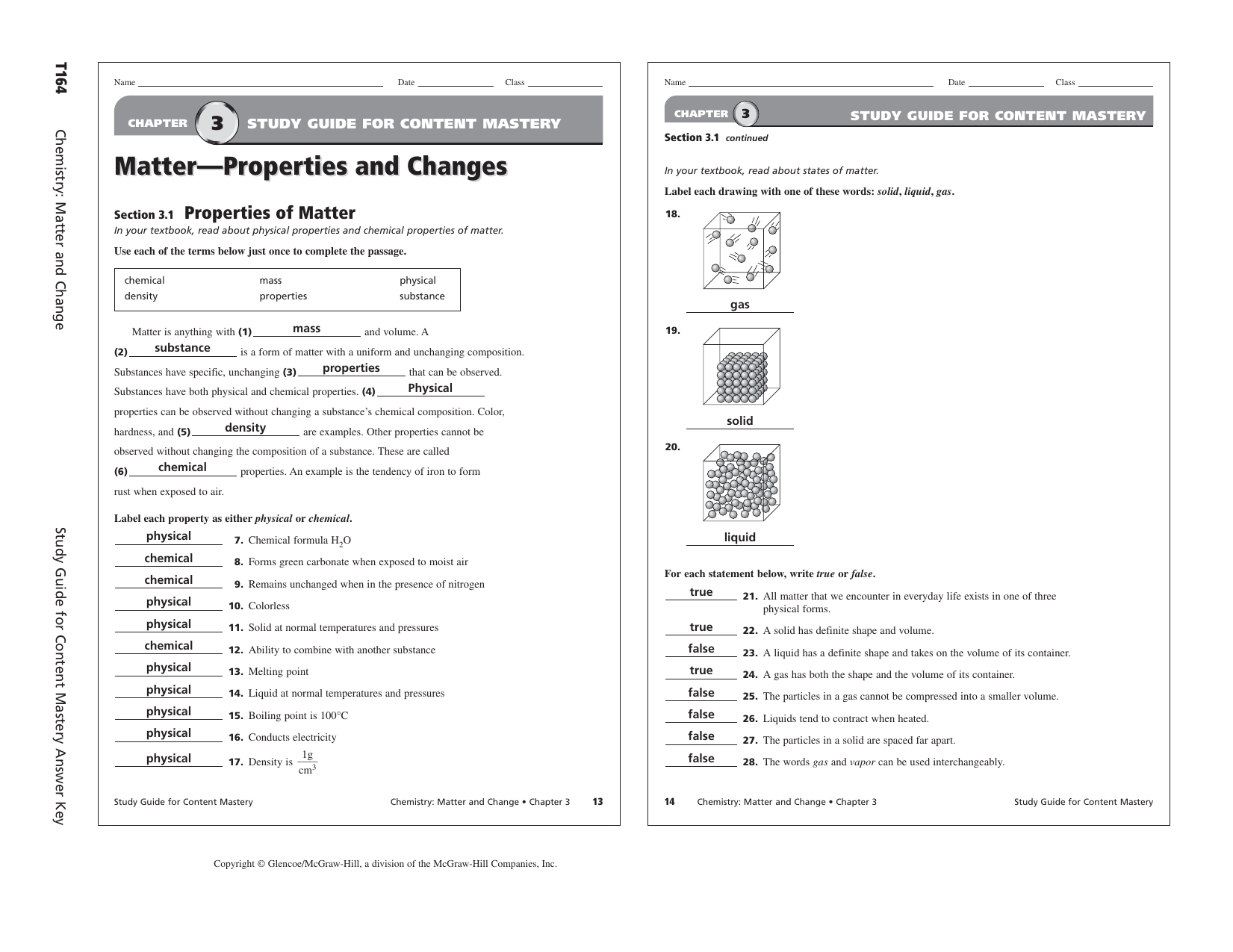
Chapter 3 Study Guide Key
Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. State the principle that explains why there must be the same number of atoms of each elements on each side of an equation. In addition, each chapter contains numerous skillbuilders, each of which is. Solid gas liquid all of these, the temperature at..
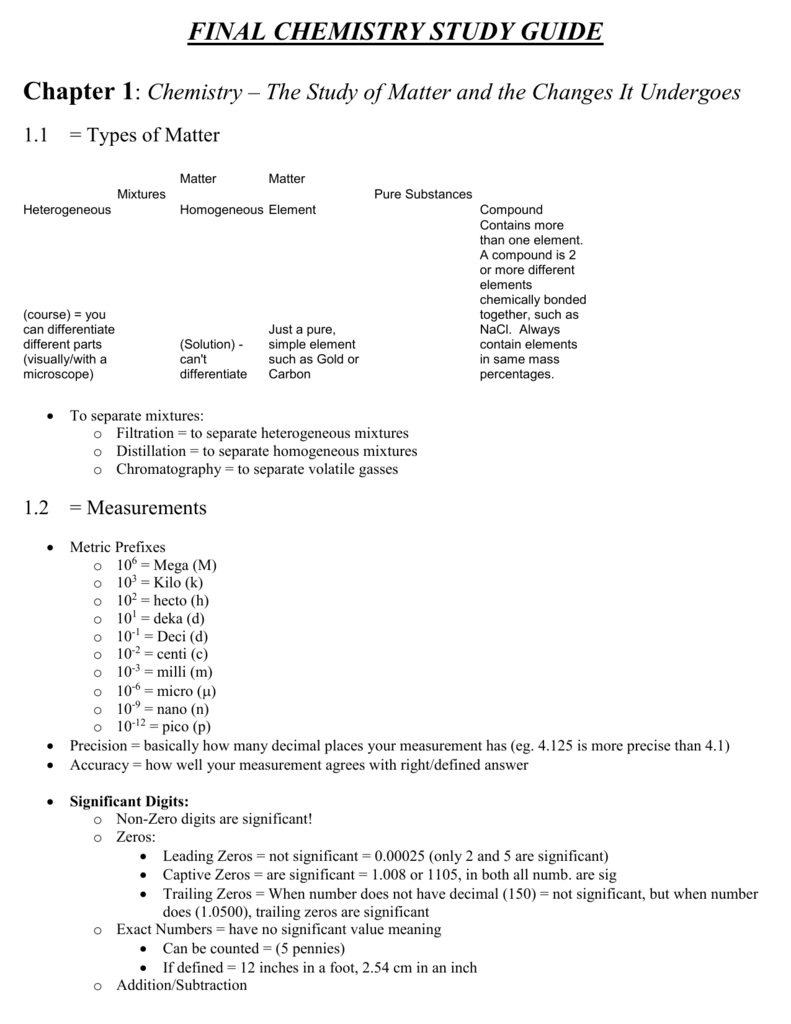
SEMESTER 1 CHEMISTRY STUDY GUIDE
Web chemistry chapter 6 study guide study flashcards learn write spell test play match gravity created by alligraz the periodic table and periodic law terms in this set (35) actinide series the periodic table,. Dissolving is a physical change and reacting is a. Web study with quizlet and memorize flashcards containing terms like chemical bond, 3 types of chemical bonds,.
PPT Chapter 6 Study Guide PowerPoint Presentation, free download ID
Solid gas liquid all of these, the temperature at. In addition, each chapter contains numerous skillbuilders, each of which is. Web chemistry chapter 6 study guide. Web study with quizlet and memorize flashcards containing terms like chemical bond, 3 types of chemical bonds, why do. Web study with quizlet and memorize flashcards containing terms like a chemical bond results from.
Chapter 6 Study Guide
Click the card to flip 👆. B) read the following sections from chapter 7 and do the problems in those sections: Electron energy level neutron nucleus proton in your. We have covered quite a number of topics up to this point: Web livingston public schools / lps homepage
Chemistry Chapter 73 Study Guide Answer Key Study Poster
Electron energy level neutron nucleus proton in your. Web a substance or molecule that participates in a chemical reaction. Web our interactive player makes it easy to find solutions to chemistry: Solid gas liquid all of these, the temperature at. Web study with quizlet and memorize flashcards containing terms like chemical bond, 3 types of chemical bonds, why do.
Study Guide for Content Mastery Teacher Edition
Label the diagram of an atom. Dissolving is a physical change and reacting is a. Web study with quizlet and memorize flashcards containing terms like chemical bond, 3 types of chemical bonds, why do. Web study with quizlet and memorize flashcards containing terms like a chemical bond results from the mutual attraction of the nuclei of atoms and _____. Electron.
Chapter 6 Study Guide
Web study with quizlet and memorize flashcards containing terms like which state of matter is characterized by having molecules close together, but moving randomly? Unit 11 states of matter and intermolecular forces. Web study with quizlet and memorize flashcards containing terms like a chemical bond results from the mutual attraction of the nuclei of atoms and _____. The structure of.
Selina Concise Chemistry Class 6 ICSE Solutions Chapter 7 Water Learn
Unit 11 states of matter and intermolecular forces. Web michigan state university and uc bolder. Web study guide chapter 6 section 2 chemical reactions. Web a substance or molecule that participates in a chemical reaction. Unit 7 electronic structure of atoms.
Label The Diagram Of An Atom.
The idea of arranging the elements in a table according to their chemical and physical properties is attributed to ________. Click the card to flip. Web this section shows how you can use the skills you learned in sections 3.6 and 6.6 and some information derived from chemical formulas for compounds to convert between mass of an element and mass of a. Web unit 6 more about chemical reactions.
Web Michigan State University And Uc Bolder.
New products atoms are not created, and old reactant atoms are not destroyed. The structure of atoms, discrete molecules, complex network solids, and metals; Web study guide chapter 6 section 2 chemical reactions. Web study with quizlet and memorize flashcards containing terms like chemical bond, 3 types of chemical bonds, why do.
Web A Substance Or Molecule That Participates In A Chemical Reaction.
Unit 10 gases and kinetic molecular theory. State the principle that explains why there must be the same number of atoms of each elements on each side of an equation. A substance that forms in a chemical reaction. In addition, each chapter contains numerous skillbuilders, each of which is.
A System That Can Exchange Mass And Energy (Usually In The Form Of Heat) With Its Surroundings.
Hit a particularly tricky question? Click the card to flip 👆. Solid gas liquid all of these, the temperature at. Explain the difference between dissolving and reacting.