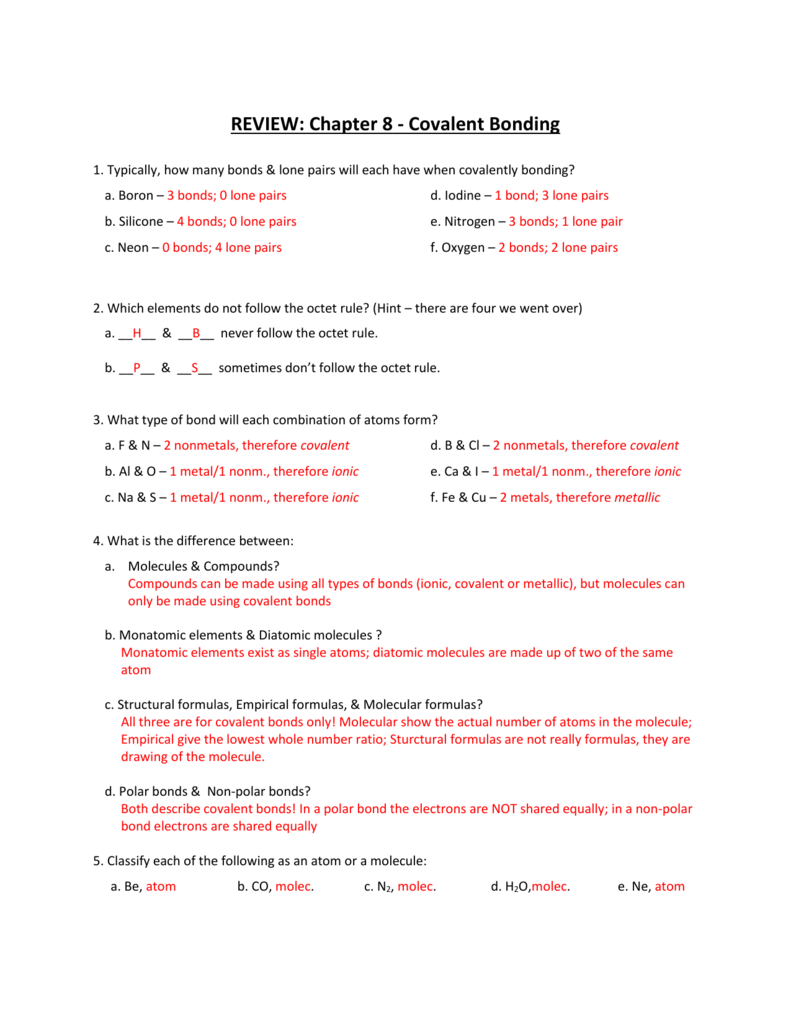
Chapter 8 Study Guide Covalent Bonding
Chapter 8 Study Guide Covalent Bonding - Neutral group of atoms joined together by covalent bonds. When covalent bonds are formed , bond dissociation energy is released and the process is. A molecule consisiting of two atoms. For example, consider a simple covalently bonded. Learn vocabulary, terms, and more with flashcards, games, and other study. Web chapter 8 study guide: Web start studying chapter 8 covalent bonding test (study guide). The energy needed to break covalent bond. Li2s kmno4 al2o3 095_112_cmc_sn_se_878755.qxd 03/21/2007 11:21 am page 96 printer pdf 2 name covalent bonding section 8.1 the covalent bond. Web chapter 8 covalent bonding study guide:
Greater amount of energy is required to break a bond in reactants than is released when the new bonds. A tightly bound group of atoms that has either a positive or negative charge and behaves as a unit. • they can’t give away electrons to bond. Learn vocabulary, terms, and more with flashcards, games, and other study. Neutral group of atoms joined together by covalent bonds. Web combinations of atoms of the nonmetals and metalloids in groups 4a, 5a, 6a, and 7a of the periodic table are likely to form covalent bonds. Click the card to flip 👆 definition 1 / 69 covalent bond click. What are all the diatomic. Bond formed between two or more atoms by a sharing of electrons metallic bond : Web shared electrons are centered between the 2 atoms in covalent bonding, the attachment is called.
Web chapter 8 covalent bonding study guide: Web chapter 8 identify the ions, along with their charges, in the following ionic compounds. A tightly bound group of atoms that has either a positive or negative charge and behaves as a unit. When h+ forms a bond with h2o the form the hydronium ion h3o+, this bond is called a coordinate covalent bond because ______. • they can’t give away electrons to bond. Molecule consisting of two of the same atoms. Bonding in which the bonding electrons are relatively free to move throughout the 3d structure electron dot. In this case the atoms usually acquire a total of eight electrons, or an octet, by sharing electrons, so that the octet rule applies. Web a bond formed by sharing three pairs of electrons. When covalent bonds are formed , bond dissociation energy is released and the process is.
Chapter 8 Study Guide Covalent Bonding Answer Key Study Poster
Web when one atom contributes both bonding electrons in a single covalent bond, the bond is called __________. Web name _____ date _____ chapter 8 study guide covalent bonding • what is a covalent bond? Web shared electrons are centered between the 2 atoms in covalent bonding, the attachment is called. A covalent bond is characterized by the sharing of.
Chemistry Chp 8 Covalent Bonding Study Guide
Click the card to flip 👆 definition 1 / 69 covalent bond click. Web combinations of atoms of the nonmetals and metalloids in groups 4a, 5a, 6a, and 7a of the periodic table are likely to form covalent bonds. Web chapter 8 identify the ions, along with their charges, in the following ionic compounds. Molecule consisting of two of the.
Chemistry Chp 8 Covalent Bonding Study Guide
Web name _____ date _____ chapter 8 study guide covalent bonding • what is a covalent bond? • what are the characteristics of molecular compounds (covalently bonded compounds) characteristics of ionic and covalent compounds characteristics ionic compound covalent compound representative element formula unit bond. Covalent bonding in this chapter: Web chapter 8 covalent bonding study guide: Web study with quizlet.
30 Covalent Bonding Worksheet Answers Education Template
A tightly bound group of atoms that has either a positive or negative charge and behaves as a unit. Web study with quizlet and memorize flashcards containing terms like what are the differences between ionic and covalent bonds?, which bonds are stronger?, which bonds have higher melting points? Web when one atom contributes both bonding electrons in a single covalent.
Chapter 8 Study Guide Covalent Bonding Answer Key Study Poster
Web combinations of atoms of the nonmetals and metalloids in groups 4a, 5a, 6a, and 7a of the periodic table are likely to form covalent bonds. The energy needed to break covalent bond. Click the card to flip 👆 definition 1 / 69 covalent bond click. Li2s kmno4 al2o3 095_112_cmc_sn_se_878755.qxd 03/21/2007 11:21 am page 96 printer pdf 2 name covalent.
30 Covalent Bonding Worksheet Answers Education Template
Web chapter 8 covalent bonding study guide: The energy needed to break covalent bond. Web covalent bonds •nonmetals hold on to their valence electrons. Covalent bonding term 1 / 69 when sharing of electrons occurs, the attachment between atoms that results is called a (n) ___. Web when one atom contributes both bonding electrons in a single covalent bond, the.
30 Covalent Bonding Worksheet Answers Education Template
Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes covalent bonds study guide has everything you need to ace quizzes, tests, and essays. A covalent bond in which one atom contributesboth bonding electrons. Click the card to flip 👆 definition 1 / 69 covalent bond click. When h+ forms a bond with h2o the.
Chemistry Chapter 8 Covalent Bonding Worksheet Answers / Ionic And
Greater amount of energy is required to break a bond in reactants than is released when the new bonds. What are all the diatomic. Web combinations of atoms of the nonmetals and metalloids in groups 4a, 5a, 6a, and 7a of the periodic table are likely to form covalent bonds. For example, consider a simple covalently bonded. Web 8 covalent.
PPT Chapter 8 Covalent Bonding PowerPoint Presentation, free
Web study with quizlet and memorize flashcards containing terms like covalent bond, dipole, double bond and more. • what are the characteristics of molecular compounds (covalently bonded compounds) characteristics of ionic and covalent compounds characteristics ionic compound covalent compound representative element formula unit bond. A covalent bond in which one atom contributesboth bonding electrons. When covalent bonds are formed ,.
PPT Covalent Bonding Chapter 8 PowerPoint Presentation, free download
Web chapter 8 covalent bonding study guide: Web a bond formed by the sharing of electrons between atoms. For example, consider a simple covalently bonded. Molecule consisting of two of the same atoms. • get it by sharing valence electrons with each other = covalent bonding.
Web 8 Covalent Bonding Basics.
What are all the diatomic. Web a bond formed by sharing three pairs of electrons. This happens most often between nonmetal elements such as carbon, hydrogen, oxygen and nitrogen. The energy needed to break covalent bond.
Bonding In Which The Bonding Electrons Are Relatively Free To Move Throughout The 3D Structure Electron Dot.
Web chapter 8 identify the ions, along with their charges, in the following ionic compounds. Web chapter 8 study guide: A covalent bond is characterized by the sharing of valence electrons by two adjacent atoms. • they can’t give away electrons to bond.
For Example, Consider A Simple Covalently Bonded.
Molecule consisting of two of the same atoms. When h+ forms a bond with h2o the form the hydronium ion h3o+, this bond is called a coordinate covalent bond because ______. Web study with quizlet and memorize flashcards containing terms like covalent bond, dipole, double bond and more. In this case the atoms usually acquire a total of eight electrons, or an octet, by sharing electrons, so that the octet rule applies.
A Covalent Bond In Which One Atom Contributesboth Bonding Electrons.
Web about this chapter this chemistry chapter on covalent bonds will go over different forces and bonding. Web when one atom contributes both bonding electrons in a single covalent bond, the bond is called __________. Covalent bonding in this chapter: Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes covalent bonds study guide has everything you need to ace quizzes, tests, and essays.