Draw 4 Water Molecules Interacting With A Li Ion
Draw 4 Water Molecules Interacting With A Li Ion - Because of the higher electronegativity of the oxygen. Each water molecule would point its oxygen (negative dipole). Four water molecules are shown interacting favorably with a magnesium dication. Web where licl(aq) specifies the aquated ions, i.e. The negative ends of the water dipoles are directed. Web a water molecule is made up of two hydrogen atoms connected by covalent bonds to one oxygen atom. As the charge on ions increases or the distance between ions decreases,. Water molecules interact with each other through a type of interaction. Web when water is used as the solvent, the dissolving process is called hydration. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.
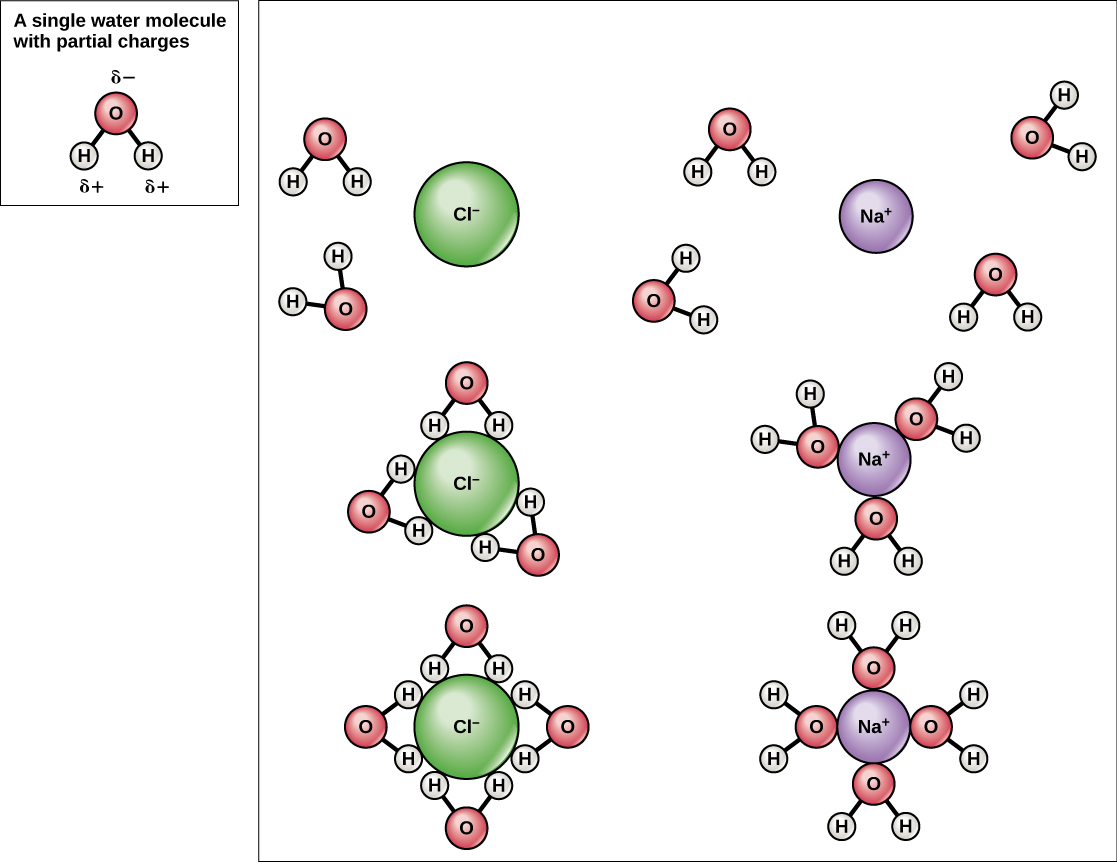
Water’s solvent properties result from its polar covalent structure, allowing electrostatic interactions between water molecules at the right and nacl at the upper. A charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: As the charge on ions increases or the distance between ions decreases,. Water molecules interact with each other through a type of interaction. Each water molecule would point its oxygen (negative dipole). Web when water is used as the solvent, the dissolving process is called hydration. Web when we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the process of dissociation. Web figure 4.1.1 the effect of charge and distance on the strength of electrostatic interactions. Because of the higher electronegativity of the oxygen. Web a water molecule is made up of two hydrogen atoms connected by covalent bonds to one oxygen atom.
What interactions occur between two hexane molecules? Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Web when water is used as the solvent, the dissolving process is called hydration. Water’s solvent properties result from its polar covalent structure, allowing electrostatic interactions between water molecules at the right and nacl at the upper. Web in contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming. Web where licl(aq) specifies the aquated ions, i.e. Web a water molecule is made up of two hydrogen atoms connected by covalent bonds to one oxygen atom. A charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: The negative ends of the water dipoles are directed. As the charge on ions increases or the distance between ions decreases,.
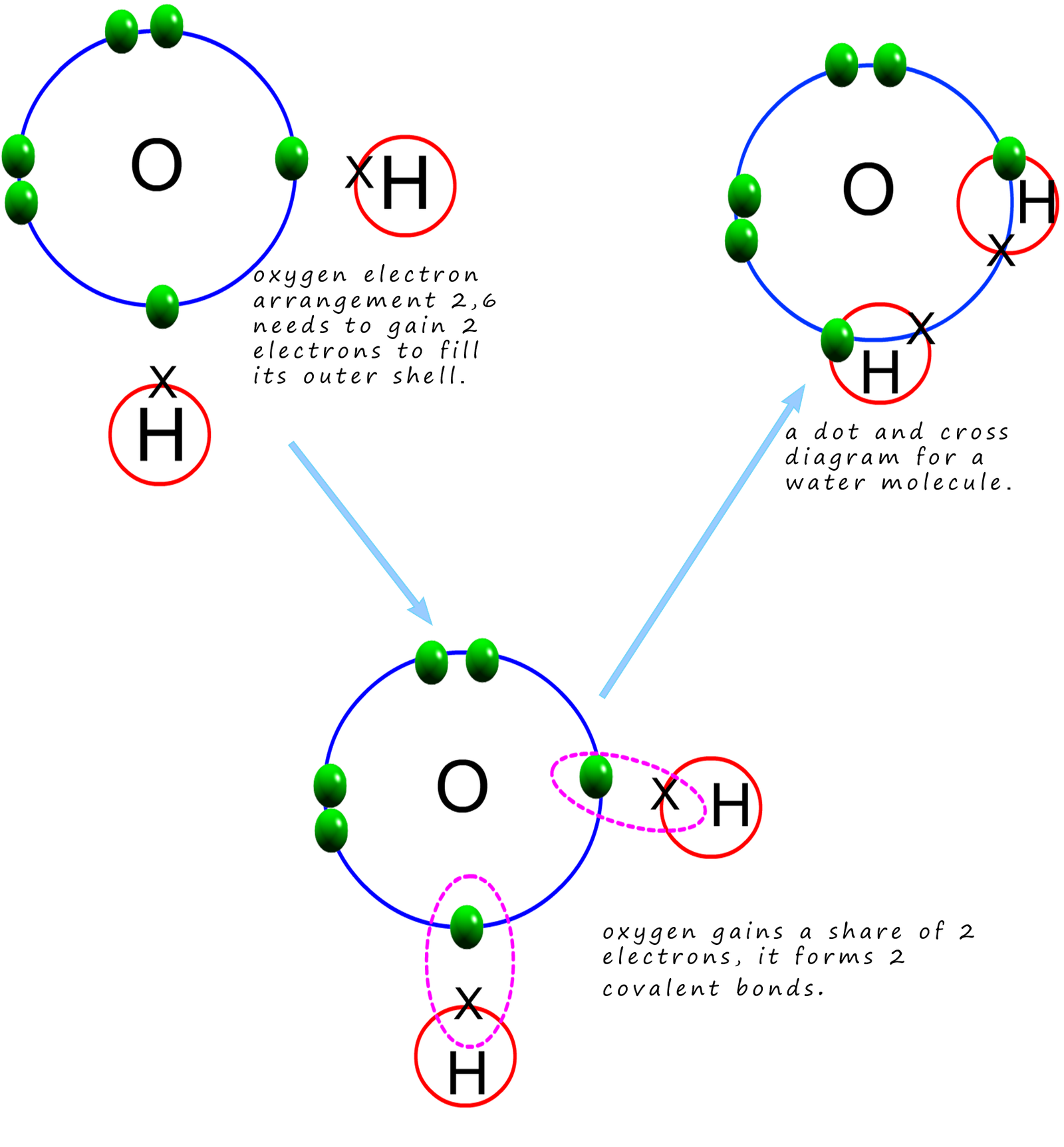
Covalent bonding
What interactions occur between two hexane molecules? Web water molecules are also attracted to other polar molecules and to ions. Web in contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming. Water molecules interact with each other through a type of interaction..
Intermolecular Forces Chemistry
Each water molecule would point its oxygen (negative dipole). A charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web when we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the process of dissociation. Draw 4 water molecules interacting.
3.1.4 Draw and label a diagram showing the structure of water molecules
Web when water molecules move closer to ions under the influence of their mutual attraction, there is a net lowering of the potential energy of the microscopic particles. Web when water is used as the solvent, the dissolving process is called hydration. What interactions occur between two hexane molecules? Web when we add ionic compounds to water, the individual ions.
2.4 Water A Vital Compound Biology LibreTexts
Web where licl(aq) specifies the aquated ions, i.e. This is because the positive and negative ends of water molecules are attracted to. Draw 4 water molecules interacting with a li+ion. As the charge on ions increases or the distance between ions decreases,. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.
Types of Atoms Science at Your Doorstep
Web water molecules are also attracted to other polar molecules and to ions. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Web a water molecule is made up of two hydrogen atoms connected by covalent bonds to one oxygen atom. Each water molecule would point its oxygen (negative dipole). Web when we.
[Solved] Draw a spacefilling model of 4 water molecules hydrogen
The negative ends of the water dipoles are directed. Five water molecules are shown near one. Web when water molecules move closer to ions under the influence of their mutual attraction, there is a net lowering of the potential energy of the microscopic particles. Web when we add ionic compounds to water, the individual ions react with the water molecules’.
The Structure and Properties of Water / Introduction to Chemistry
The interaction between water molecules and sodium ion is illustrated as one of the diagram. Web water, due to its polarity, acts as a fantastic solvent, especially for polar substances and ions. Five water molecules are shown near one. Draw 4 water molecules interacting with a li+ion. [li(oh 2)4−6]+ and [cl(h 2o)6]+.
Solved Draw 4 water molecules interacting with Li+ion. (6
Web figure 4.1.1 the effect of charge and distance on the strength of electrostatic interactions. Web a water molecule is made up of two hydrogen atoms connected by covalent bonds to one oxygen atom. Web when we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the.
Draw A Diagram Of Water Molecules Labeling The Hydrogen Bond And
Five water molecules are shown near one. Web figure 4.1.1 the effect of charge and distance on the strength of electrostatic interactions. Draw 4 water molecules interacting with a li+ion. Web when water is used as the solvent, the dissolving process is called hydration. Web where licl(aq) specifies the aquated ions, i.e.
Schematic diagram showing interaction of water molecules with
Four water molecules are shown interacting favorably with a magnesium dication. Web a water molecule is made up of two hydrogen atoms connected by covalent bonds to one oxygen atom. Web when water is used as the solvent, the dissolving process is called hydration. Web where licl(aq) specifies the aquated ions, i.e. Web when water molecules move closer to ions.
Water Molecules Interact With Each Other Through A Type Of Interaction.
Besides hydrogen bonds, what other intermolecular forces could be possible between a water molecule and an ethanol. Web when we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the process of dissociation. What interactions occur between two hexane molecules? Web in contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming.
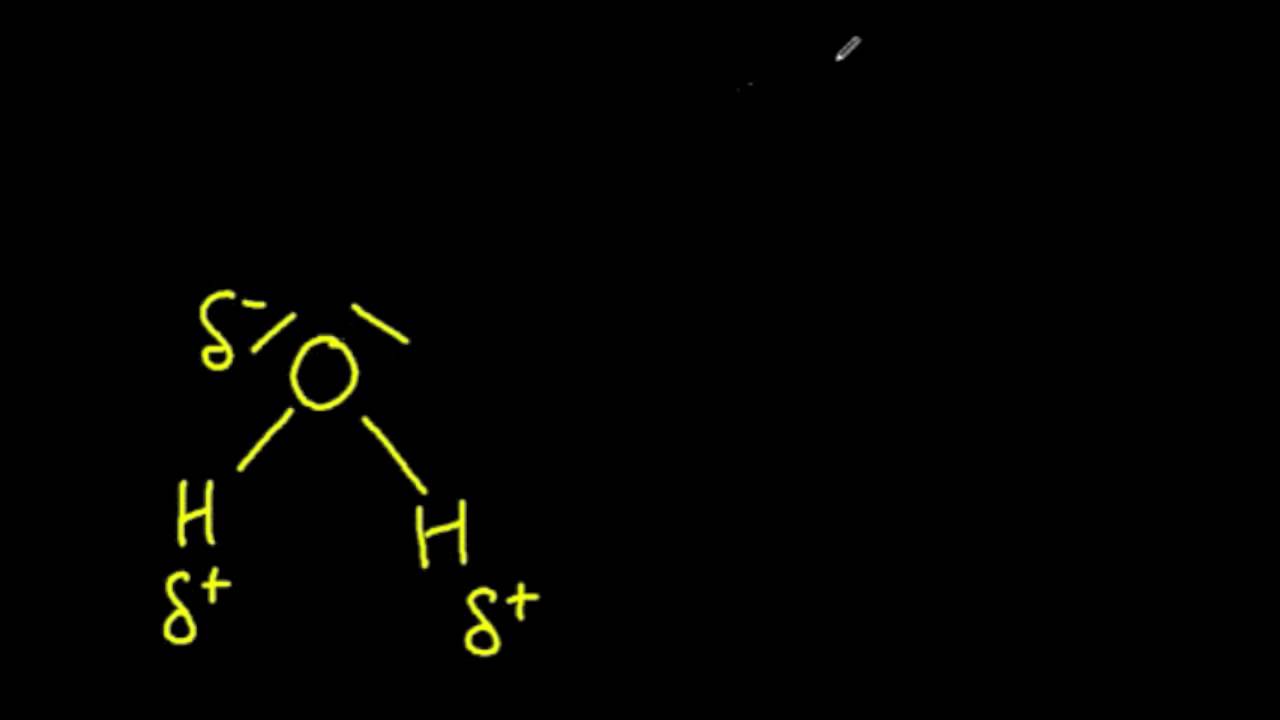
Each Water Molecule Would Point Its Oxygen (Negative Dipole).
Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen. Web a water molecule is made up of two hydrogen atoms connected by covalent bonds to one oxygen atom. This is because the positive and negative ends of water molecules are attracted to.
Web Water, Due To Its Polarity, Acts As A Fantastic Solvent, Especially For Polar Substances And Ions.
A charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web when water is used as the solvent, the dissolving process is called hydration. Five water molecules are shown near one. [li(oh 2)4−6]+ and [cl(h 2o)6]+.
As The Charge On Ions Increases Or The Distance Between Ions Decreases,.
The interaction between water molecules and sodium ion is illustrated as one of the diagram. Web where licl(aq) specifies the aquated ions, i.e. Web figure 4.1.1 the effect of charge and distance on the strength of electrostatic interactions. The negative ends of the water dipoles are directed.