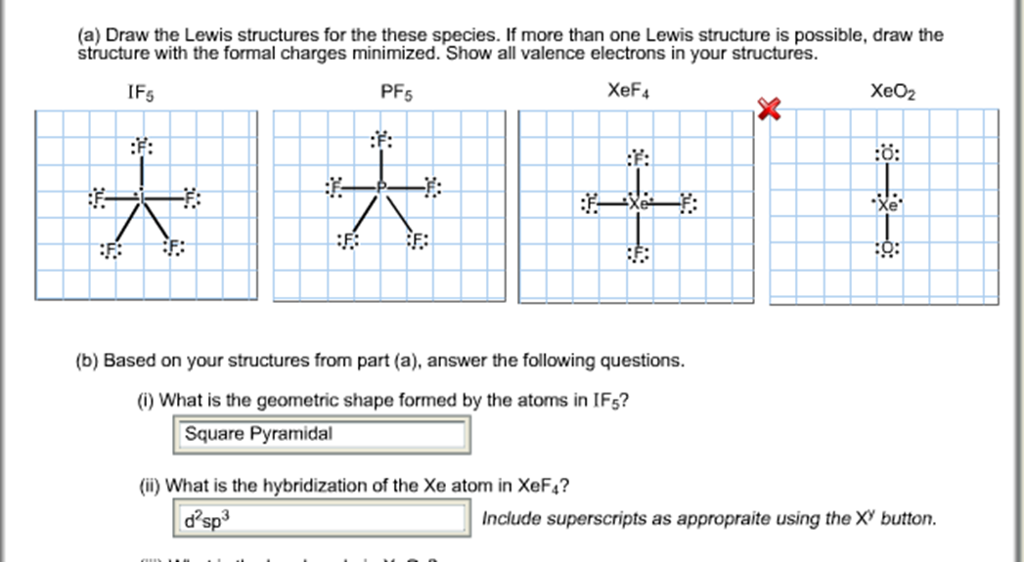
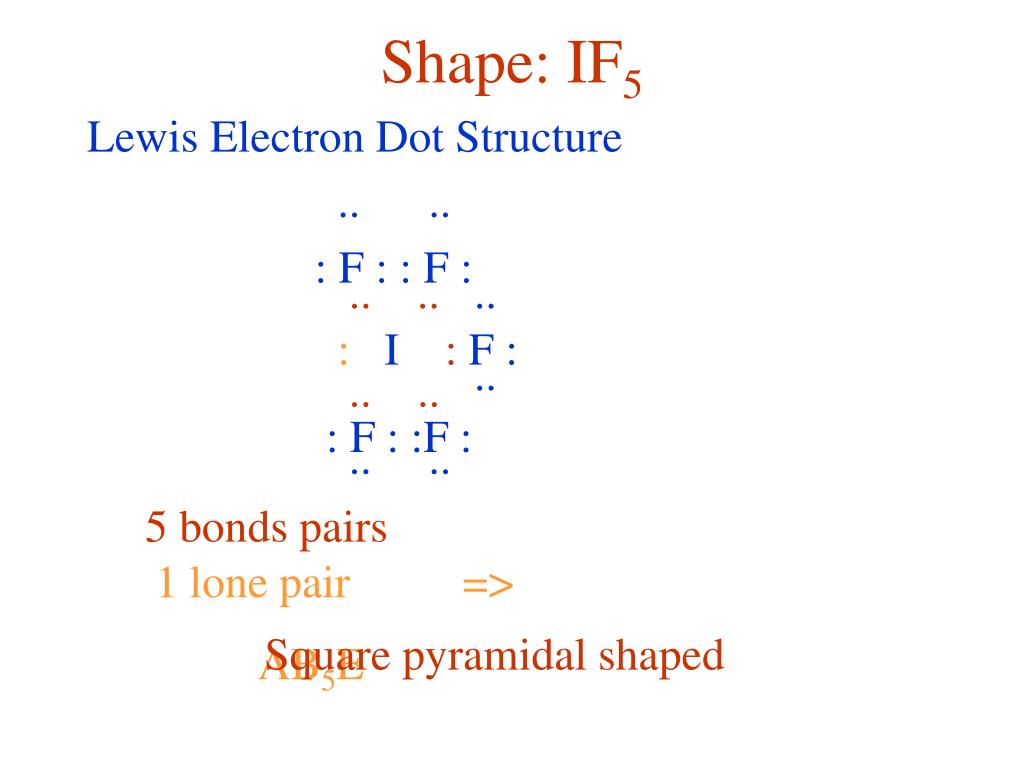
Draw An Appropriate Lewis Structure For If5
Draw An Appropriate Lewis Structure For If5 - Put one electron pair in each bond4. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Let us follow a few steps. Web i quickly take you through how to draw the lewis structure of if5 (iodine pentafluoride). (a) draw an appropriate lewis structure, (b) sketch the molecule with an accurate representation of the geometry, indicating bond angles, (c) name the molecular geometry for each central atom, (d) determine whether the molecule is polar, and (e) identify the hybridiz. The if 5 lewis structure you'll need to put more than eight valence electrons on the iodine atom. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Send feedback | visit wolfram|alpha. Draw (on paper) a lewis structure for if_5 and answer the following questions based on your drawing. This widget gets the lewis structure of chemical compounds.
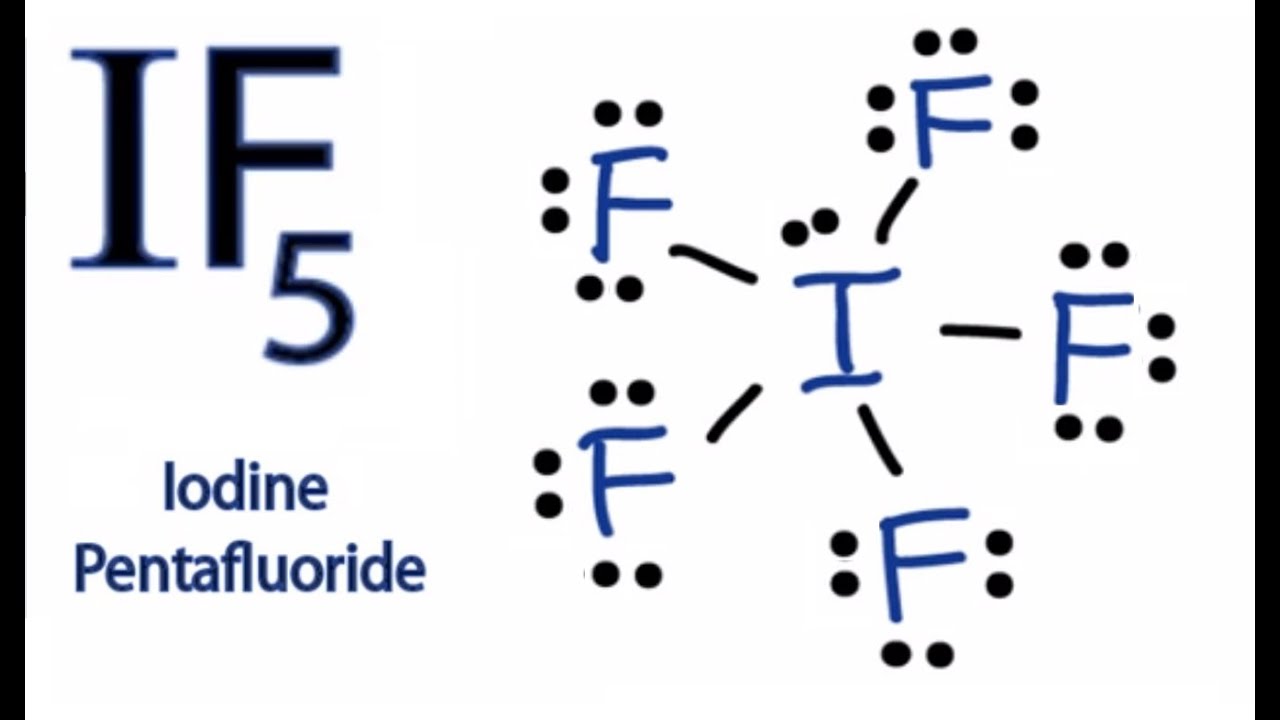
The iodine atom has 1 lone pair while all the five fluorine atoms have 3 lone pairs. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. By the end of this section, you will be able to: The if 5 lewis structure you'll need to put more than eight valence electrons on the iodine atom. We draw lewis structures to predict: I also go over hybridization, shape and bond angle. #1 draw a rough sketch of the structure. Draw lewis structures depicting the bonding in simple molecules. Write lewis symbols for neutral atoms and ions. Draw (on paper) a lewis structure for if_5 and answer the following questions based on your drawing.
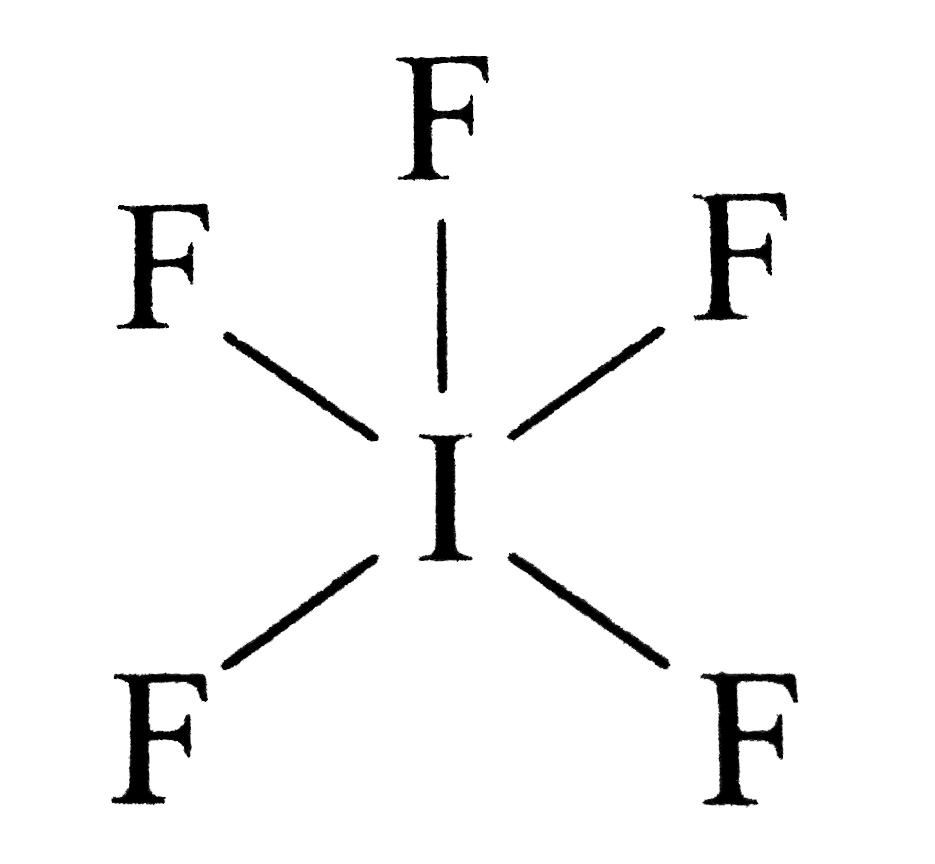
#1 draw a rough sketch of the structure. Draw lewis structures depicting the bonding in simple molecules. Part a draw an appropriate lewis structure for ifs. Write the correct skeletal structure for the molecule. #1 draw a rough sketch of the structure. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. For the compounds (i) if5 and (ii) ch3sh, complete the following: Iodine pentafluoride (if5) has 5 fluorine atoms bonded to a central iodine, and that iodine has one lone pair on it. Let’s draw and understand this lewis dot structure step by step. This widget gets the lewis structure of chemical compounds.
If5 Lewis Structure
The hybridization of if5 is sp3d2. First, determine the total number of valence. Draw lewis structures depicting the bonding in simple molecules. Include all lone q (5 ଡ bo identify the geometry of if5 using vsepr theory. When drawing the structure of an ion, be sure to add/subtract electrons to account for.
If5 Lewis Structure
#1 draw a rough sketch of the structure. Let’s draw and understand this lewis dot structure step by step. The iodine atom (i) is at the center and it is surrounded by 5 fluorine atoms (f). Draw lewis structures depicting the bonding in simple molecules. Web first, we need to draw the lewis structure of if5.
IF5 Lewis Structure (Iodine Pentafluoride) YouTube
Include all lone q (5 ଡ bo identify the geometry of if5 using vsepr theory. #3 indicate formal charges on the atoms, if necessary. In the lewis structure for if 5 there are a total of 42 valence electrons. Web in the if 5 lewis structure iodine (i) is the least electronegative atom and goes in the center of the.
How To Draw Lewis Structures A Step By Step Tutorial
Draw the molecule by placing atoms on the grid and connecting them with bonds. In short, these are the steps you need to follow for drawing a lewis structure: This problem has been solved! For the central iodine atom: The if 5 lewis structure you'll need to put more than eight valence electrons on the iodine atom.
If5 Lewis Structure
#3 indicate formal charges on the atoms, if necessary. Web a video explanation of how to draw the lewis dot structure for iodine pentafluoride, along with information about the compound including formal charges, pola. This gives it ax5e1 shape. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. This problem has been.
If5 Lewis Structure
Find more chemistry widgets in wolfram|alpha. Web a video explanation of how to draw the lewis dot structure for iodine pentafluoride, along with information about the compound including formal charges, pola. By the end of this section, you will be able to: In the lewis structure for if 5 there are a total of 42 valence electrons. Web chemistry questions.
So far, we’ve used 42 of the IF5 Lewis structure’s total 42 outermost
The iodine atom has 1 lone pair while all the five fluorine atoms have 3 lone pairs. Iodine pentafluoride (if5) has 5 fluorine atoms bonded to a central iodine, and that iodine has one lone pair on it. Added jun 9, 2014 by webtester in chemistry. Web steps of drawing if5 lewis structure. Figure out how many electrons the molecule.
IF5 Molecular Geometry, Bond Angles and Electron Geometry YouTube
In the lewis structure for if 5 there are a total of 42 valence electrons. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. In order to find the total valence electrons in a if5 (iodine pentafluoride) molecule, first of all you should know the valence electrons present in iodine atom as well.
Draw the Lewis structure of iodine pentafluoride, IF5.
Include all lone q (5 ଡ bo identify the geometry of if5 using vsepr theory. Web chemistry questions and answers. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Send feedback | visit wolfram|alpha. Write lewis symbols for neutral atoms and ions.
IF5 Lewis Structure How to Draw the Lewis Structure for IF5 YouTube
This gives it ax5e1 shape. The if 5 lewis structure you'll need to put more than eight valence electrons on the iodine atom. Find more chemistry widgets in wolfram|alpha. First, determine the total number of valence. Draw the molecule by placing atoms on the grid and connecting them with bonds.
In Short, These Are The Steps You Need To Follow For Drawing A Lewis Structure:
Web lewis structure of if5 contains five single bonds between the iodine (i) atom and each fluorine (f) atom. Which has the larger bond angle? The iodine atom (i) is at the center and it is surrounded by 5 fluorine atoms (f). #1 draw a rough sketch of the structure.
Web Thus The Lewis Structure Of Any Compound Can Be Formed Using These Simple Steps!
#2 next, indicate lone pairs on the atoms. The number of lone pairs = the number of single bonds = the number of double bonds = 2. Draw the molecule by placing atoms on the grid and connecting them with bonds. Iodine pentafluoride (if5) has 5 fluorine atoms bonded to a central iodine, and that iodine has one lone pair on it.
Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
Web lewis structure for if5. By the end of this section, you will be able to: This problem has been solved! Draw lewis structures depicting the bonding in simple molecules.
This Gives It Ax5E1 Shape.
(a) draw an appropriate lewis structure, (b) sketch the molecule with an accurate representation of the geometry, indicating bond angles, (c) name the molecular geometry for each central atom, (d) determine whether the molecule is polar, and (e) identify the hybridiz. Put least electronegative atom in centre3. Draw (on paper) a lewis structure for if_5 and answer the following questions based on your drawing. We draw lewis structures to predict: