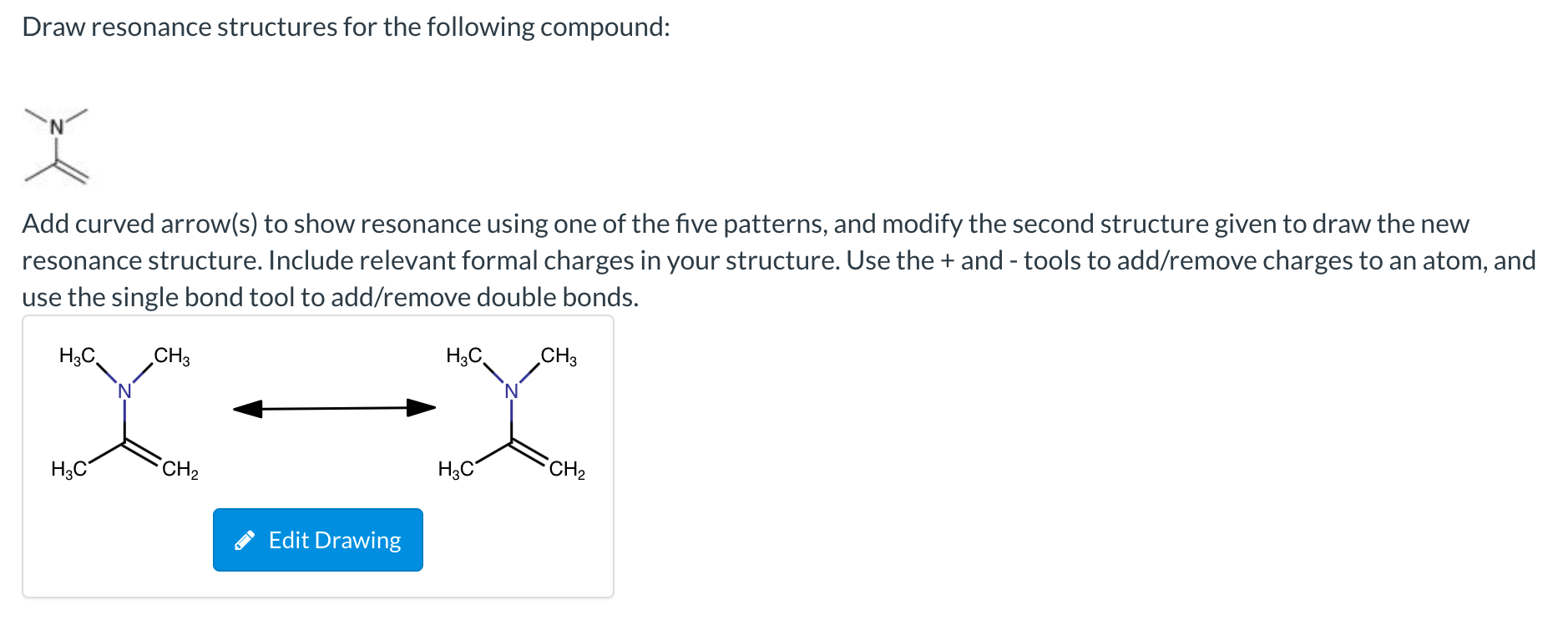
Draw Resonance Structures For The Following Compound
Draw Resonance Structures For The Following Compound - There is only one π bond in this example, and no any lone pairs, so only the π electrons can be. (iii) c h 2 = c h − c | h = o. Ozone (o3) ozone has two major resonance structures that contribute equally to its overall hybrid structure. (i) c h 2 = c h − c l. When switching from general to organic chemistry, showing. Resonance is a mental exercise and method. Web it explains how to draw the resonance structures using curved arrow notation and how to draw the resonance hybrid as well with all of the lone pairs, bonds, and partial charges. Web draw the resonance structures of the following compounds: Not all will obey the octet rule. To draw all resonance structures, take the lewis structure we drawn by using vespr rule.
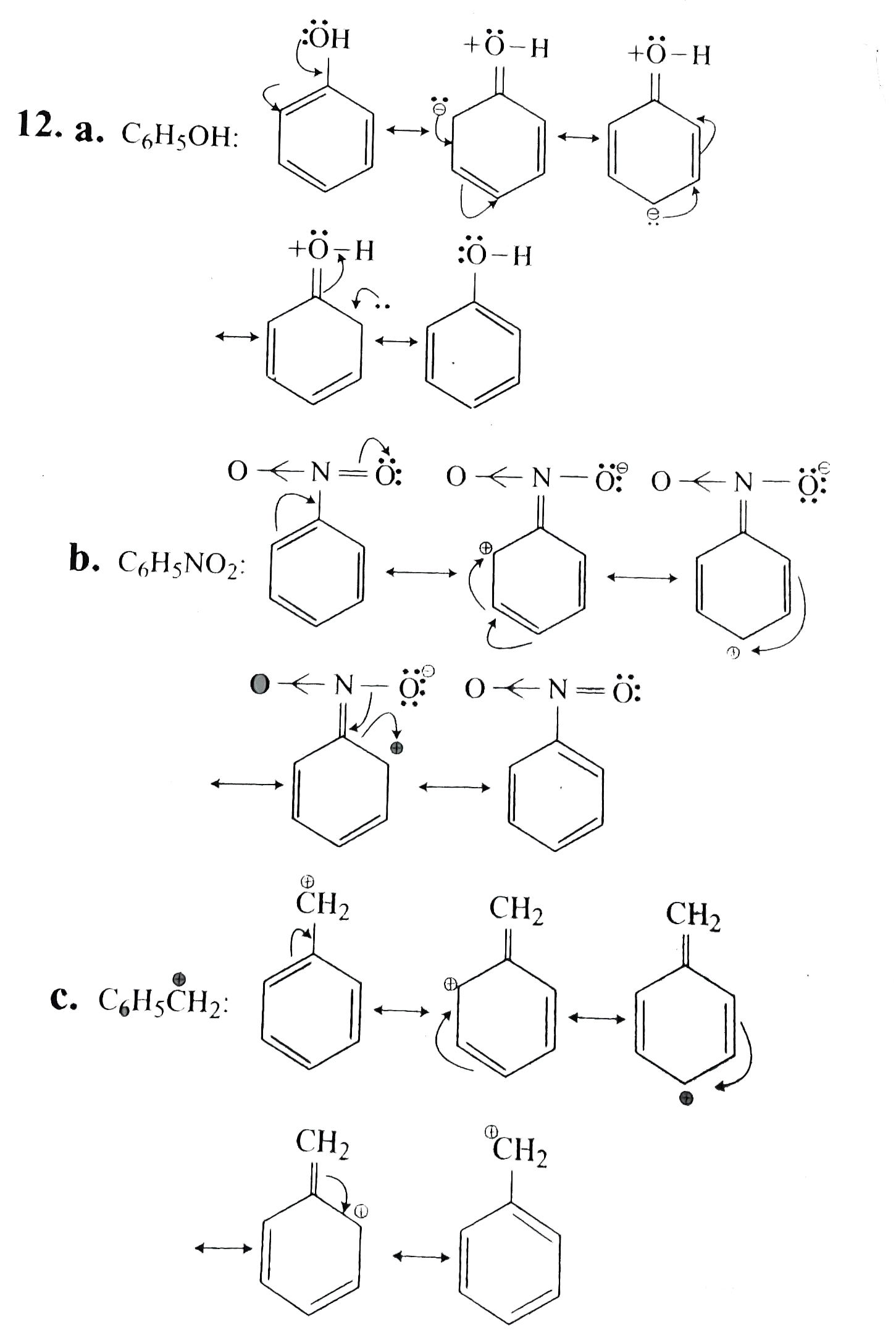
To draw all resonance structures, take the lewis structure we drawn by using vespr rule. Web it explains how to draw the resonance structures using curved arrow notation and how to draw the resonance hybrid as well with all of the lone pairs, bonds, and partial charges. (iii) c h 2 = c h − c | h = o. (ii) c h 2 = c h − c h = c h 2. Not all will obey the octet rule. In resonance structures, it does not require to show. Draw another resonance structure based on the given one. Correct option is a) solve any question of. Ozone (o3) ozone has two major resonance structures that contribute equally to its overall hybrid structure. Web (a) the structure of c 6 h 5 oh is:
Ozone (o3) ozone has two major resonance structures that contribute equally to its overall hybrid structure. The following rules should be helpful: Not all will obey the octet rule. (ii) c h 2 = c h − c h = c h 2. Each oxygen atom has 6 valence electrons, making it a total of. The resonating structures of nitro benzene are. (b) the structure of c 6 h 5 no 2 is: Web draw all possible resonance structures for the following free radical: A pi bond between two atoms. Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule.
[Solved] Draw resonance structures for the follow
The resonating structures of nitro benzene are. A pi bond between two atoms. Calculate the total number of valence electrons from each atom. Web when first dealing with resonance forms, it’s useful to have a set of guidelines that describe how to draw and interpret them. Not all will obey the octet rule.
SOLVED Draw all significant resonance structures for the following
Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. To draw all resonance structures, take the lewis structure we drawn by using vespr rule. Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. Web draw the resonance structures of the following compounds:.
Draw a Resonance Structure for the Compound Below.
Not all will obey the octet rule. Determine all the pushable electron pairs and the places where the electrons. A pi bond between two atoms. In many cases, a single. Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule.
[Solved] . Draw resonance structures for the following compounds. Add
Draw sets of resonance structures for the following compounds. Web how to draw resonance structures. In resonance structures, it does not require to show. When switching from general to organic chemistry, showing. Determine all the pushable electron pairs and the places where the electrons.
1.3 Resonance Structures Organic Chemistry I
Web draw all possible resonance structures for the following free radical: To draw all resonance structures, take the lewis structure we drawn by using vespr rule. When switching from general to organic chemistry, showing. Draw another resonance structure based on the given one. Draw sets of resonance structures for the following compounds.
Solved Draw resonance structures for the following compound
Use curved arrows to depict the conversion. Web draw the resonance structures of the following compounds; Web draw all possible resonance structures for the following free radical: To draw all resonance structures, take the lewis structure we drawn by using vespr rule. Each oxygen atom has 6 valence electrons, making it a total of.
draw significant resonance structures for the following compound
The resonating structures of phenol are represented as: Calculate the total number of valence electrons from each atom. (b) the structure of c 6 h 5 no 2 is: Web when first dealing with resonance forms, it’s useful to have a set of guidelines that describe how to draw and interpret them. Some molecules have two or more chemically equivalent.
Solved Draw resonance structures for the following compound
Web how to draw resonance structures. When switching from general to organic chemistry, showing. (i) c h 2 = c h − c l. Each oxygen atom has 6 valence electrons, making it a total of. The resonating structures of nitro benzene are.
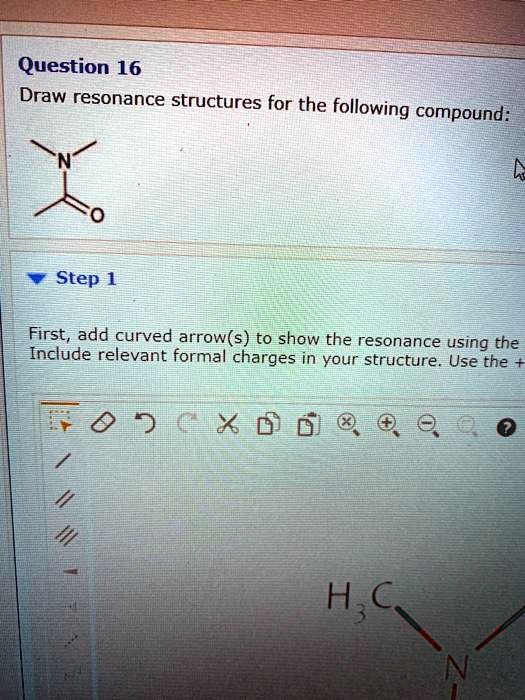
SOLVED Question 16 Draw resonance structures for the following
Draw another resonance structure based on the given one. Ozone (o3) ozone has two major resonance structures that contribute equally to its overall hybrid structure. When switching from general to organic chemistry, showing. (ii) c h 2 = c h − c h = c h 2. Web draw the resonance structures of the following compounds;
Draw the resonance structures for the following compounds. Show the el
Web draw the resonance structures of the following compounds: Use curved arrows to depict the conversion. There is only one π bond in this example, and no any lone pairs, so only the π electrons can be. Web it explains how to draw the resonance structures using curved arrow notation and how to draw the resonance hybrid as well with.
Web How To Draw Resonance Structures.
A pi bond between two atoms. The resonating structures of phenol are represented as: Web (a) the structure of c 6 h 5 oh is: Resonance is a mental exercise and method.
Each Oxygen Atom Has 6 Valence Electrons, Making It A Total Of.
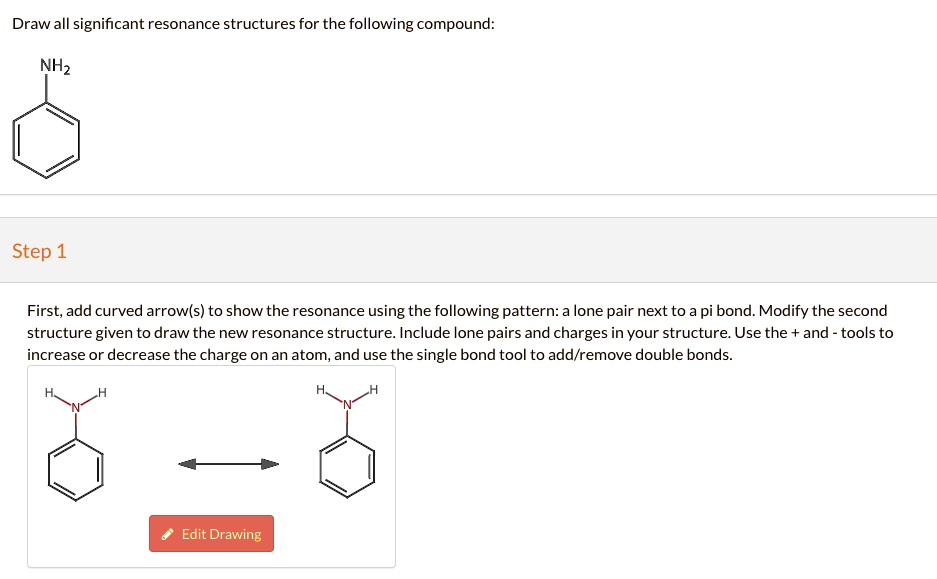
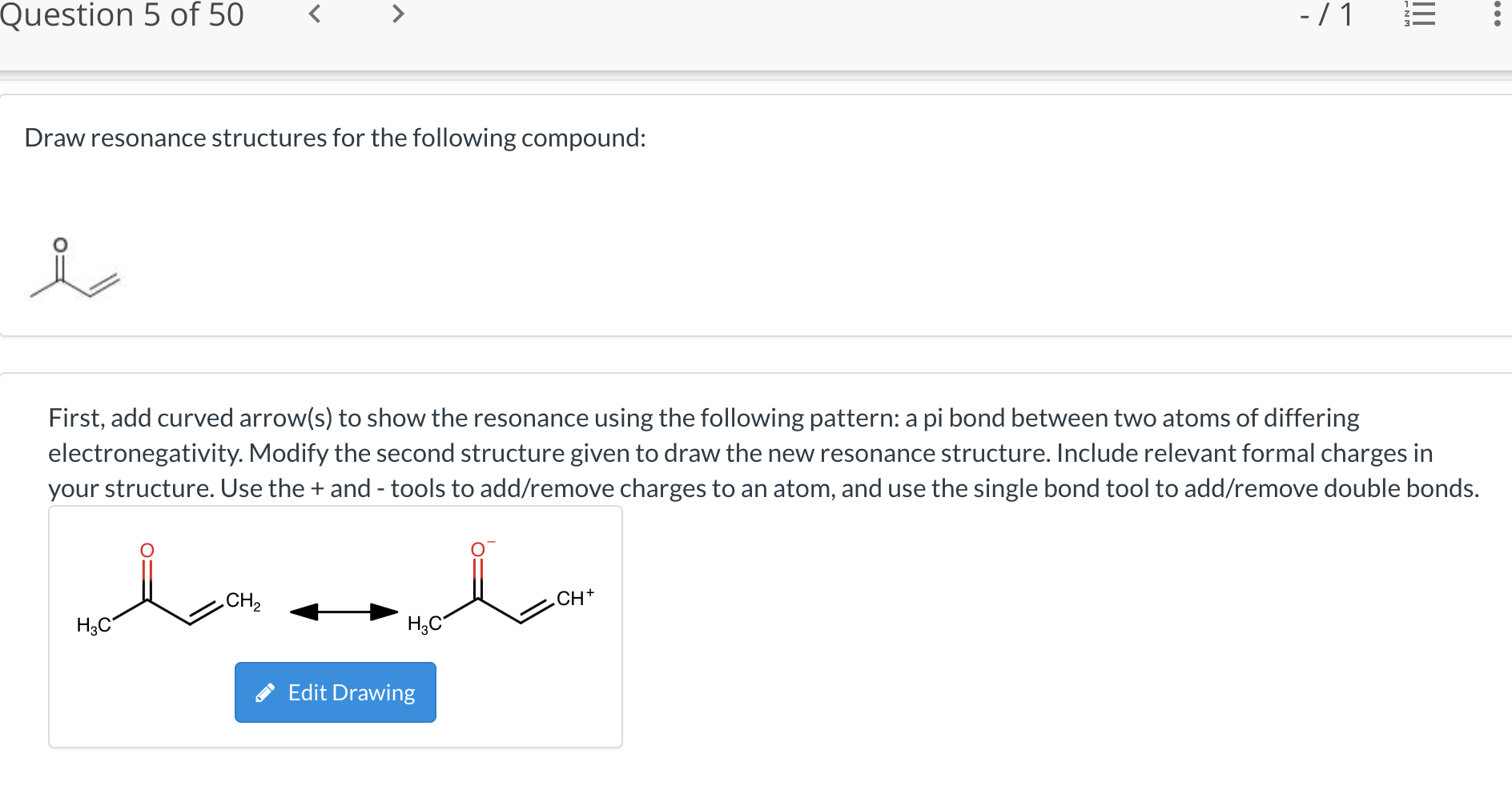
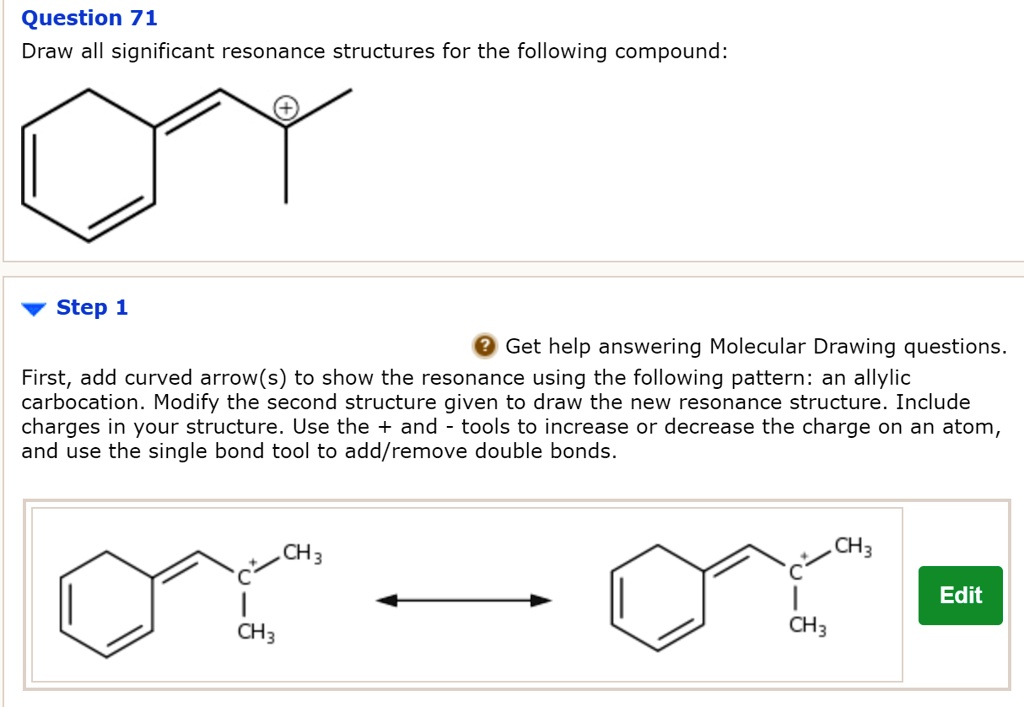
Not all will obey the octet rule. Web when first dealing with resonance forms, it’s useful to have a set of guidelines that describe how to draw and interpret them. Use curved arrows to depict the conversion. Step 1 first, add curved arrows) to show the resonance using the following pattern:
When Switching From General To Organic Chemistry, Showing.
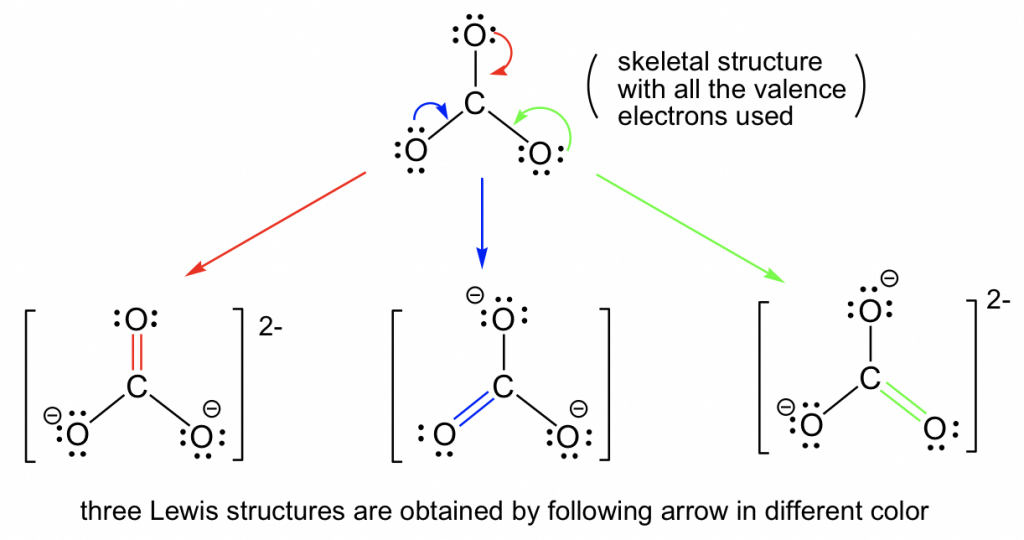
Web draw all possible resonance structures for the following free radical: Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. Web it is a superposition, in which a single molecule can behave like all three structures at the same time. Calculate the total number of valence electrons from each atom.
Web Draw The Resonance Structures Of The Following Compounds;
Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. In resonance structures, it does not require to show. Determine all the pushable electron pairs and the places where the electrons. There is only one π bond in this example, and no any lone pairs, so only the π electrons can be.
![[Solved] Draw resonance structures for the follow](https://media.cheggcdn.com/study/abf/abfbc7e3-78c5-42b6-bd2d-d0b05735ba03/image.jpg)