Draw The Lewis Structure For So3
Draw The Lewis Structure For So3 - Follow these simple steps to draw lewis dot structures: (valence electrons are the number of electrons present in the outermost shell of an atom). Now have a look of lewis structure again; Web drawing lewis dot structures and resonance structures. Web the so3 lewis structure shows a central sulfur (s) atom with three oxygen (o) atoms around it. In order to draw the lewis structure of so3, first of all you have to find the total number of valence electrons present in the so3 molecule. All atoms have sp 2 hybridization. It is a form of pollution. Web drawing the lewis structure for so 3 ( sulfur trioxide) so 3 is the primary contributer to acid rain in the atomsphere. Why so3 forms double bonds?
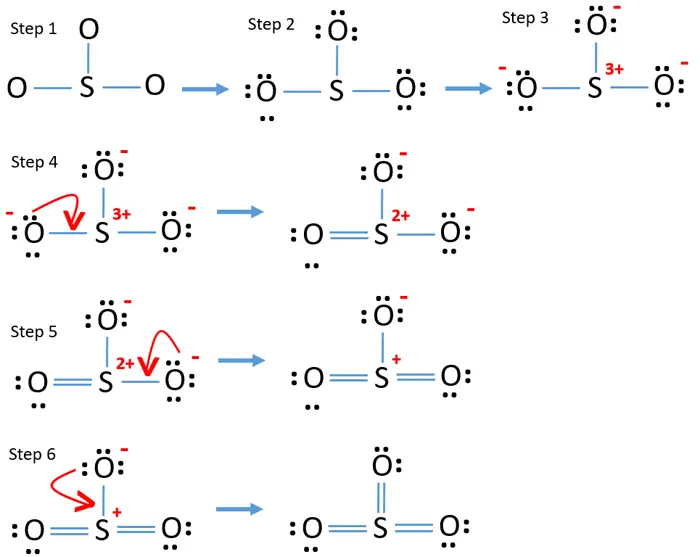
So 3 has 24 valence electrons. Put two electrons between the atoms to form chemical bonds. Follow these simple steps to draw lewis dot structures: Web the lewis structure of sulfur trioxide (so3) molecule is drawn by: So 3 is named sulfur trioxide. Web there are three resonance structures so3 (sulfur trioxide). There are 32 valence electrons available for the lewis structure for so 3. Why so3 forms double bonds? Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Remember, sulfur is in period 3 and can hold more than 8 valence electrons.
All atoms have sp 2 hybridization. Finally, draw the skeleton as: Web drawing lewis dot structures and resonance structures. Drawing the lewis structure of so3: Hybridization of so 3 molecule. Let's put the sulfur at the center and the oxygens around the outside. Web drawing the lewis structure for so 3. Why so3 forms double bonds? When we draw it, firstly we get the three structures at the top. A double bond forms between sulfur and one oxygen, satisfying the octet rule and yielding a formal charge of 0 for all atoms.
SO3 2 Lewis Structure How to Draw the Lewis Structure for SO3 2
It is a form of pollution. Let's put the sulfur at the center and the oxygens around the outside. There are seven resonance structures for so_3. There are three double bonds around sulfur atom with oxygen atoms in so molecule. Look for the central atom, which will be sulfur in this case.
Draw The Lewis Dot Structure For So3 2 slidesharedocs
Group 16 element, 6 valence. Both methanol and ethanol produce co 2 and h 2 o when they burn. There are 32 valence electrons available for the lewis structure for so 3. Look for the central atom, which will be sulfur in this case. Drawing the lewis structure of so3:
SO3 Lewis Structure, Molecular Geometry, and Hybridization
Web the lewis structure of so3 involves 24 valence electrons distributed among sulfur and three oxygen atoms. Write the chemical equations for these combustion reactions using lewis structures instead of chemical formulas. So 3 is named sulfur trioxide. Within the so 3 lewis structure, the sulfur atom is bonded to three oxygen atoms through double bonds. 6 + (3 x.
SO3 Lewis Structure, Molecular Geometry, and Hybridization
Hybridization of so 3 molecule. Why so3 forms double bonds? Note that so3 is a bi. Some molecules or ions cannot be adequately described by a single lewis structure. We start with a valid lewis structure and then follow these general rules.
SO3 Lewis StructureLewis structure of SO3 (Sulfur trioxide) YouTube
Follow these simple steps to draw lewis dot structures: So 3 is named sulfur trioxide. Web the lewis structure of sulfur trioxide (so3) molecule is drawn by: It discusses the molecular geometry, bond angle, hybridization, and. Now have a look of lewis structure again;
Draw the Lewis dot structure for SO3 Brainly.in
Methanol, h 3 coh, is used as the fuel in some race cars. Let's put the sulfur at the center and the oxygens around the outside. When we draw it, firstly we get the three structures at the top. Web the so3 lewis structure shows a central sulfur (s) atom with three oxygen (o) atoms around it. Web the so.
draw the lewis structure for so3 and answer the following questions
Each oxygen atom has two lone pairs of electrons associated with it. (valence electrons are the number of electrons present in the outermost shell of an atom). So 3 has 24 valence electrons. Web the lewis structure of so3 involves 24 valence electrons distributed among sulfur and three oxygen atoms. Web drawing lewis dot structures and resonance structures.
steps of drawing SO3 lewis structure VSEPR method
So 3 has 24 valence electrons. Follow these simple steps to draw lewis dot structures: It is a form of pollution. These atoms are connected by double bonds, and each oxygen atom has two lone pairs of electrons. Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle.
SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur
Now have a look of lewis structure again; Ethanol, c 2 h 5 oh, is used extensively as motor fuel in brazil. Web the lewis structure of so3 involves 24 valence electrons distributed among sulfur and three oxygen atoms. Methanol, h 3 coh, is used as the fuel in some race cars. We start with a valid lewis structure and.
For Example, Drawing One Lewis Structure For Ozone (O 3) Gives Us A Misleading Picture Of The Actual Bonding In The Molecule.if We Draw A Lewis Structure For O 3 (Ozone), We Get This:.
The final answer must have this number of electrons‼! Web there are three resonance structures so3 (sulfur trioxide). Be sure to check the formal charges for the lewis structure for so 3. Draw the atoms on paper and put dots around them to represent valence electrons of the atom.
Web Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
Web drawing lewis dot structures and resonance structures. 6 + (3 x 6) = 24. Let's put the sulfur at the center and the oxygens around the outside. Be sure to have the correct number of electrons.
Write The Chemical Equations For These Combustion Reactions Using Lewis Structures Instead Of Chemical Formulas.
Why so3 forms double bonds? Remember, sulfur is in period 3 and can hold more than 8 valence electrons. 4 + (3 × 6) + 2 = 24 electrons. Web the so 3 lewis structure illustrates how the atoms of sulfur trioxide, a compound composed of one sulfur atom and three oxygen atoms, are arranged.
It Discusses The Molecular Geometry, Bond Angle, Hybridization, And.
Because of equal formal charge. The lewis structure for so 3 is requires you to place more than 8 valence electrons on sulfur (s). Drawing the lewis structure of so3: Some molecules or ions cannot be adequately described by a single lewis structure.