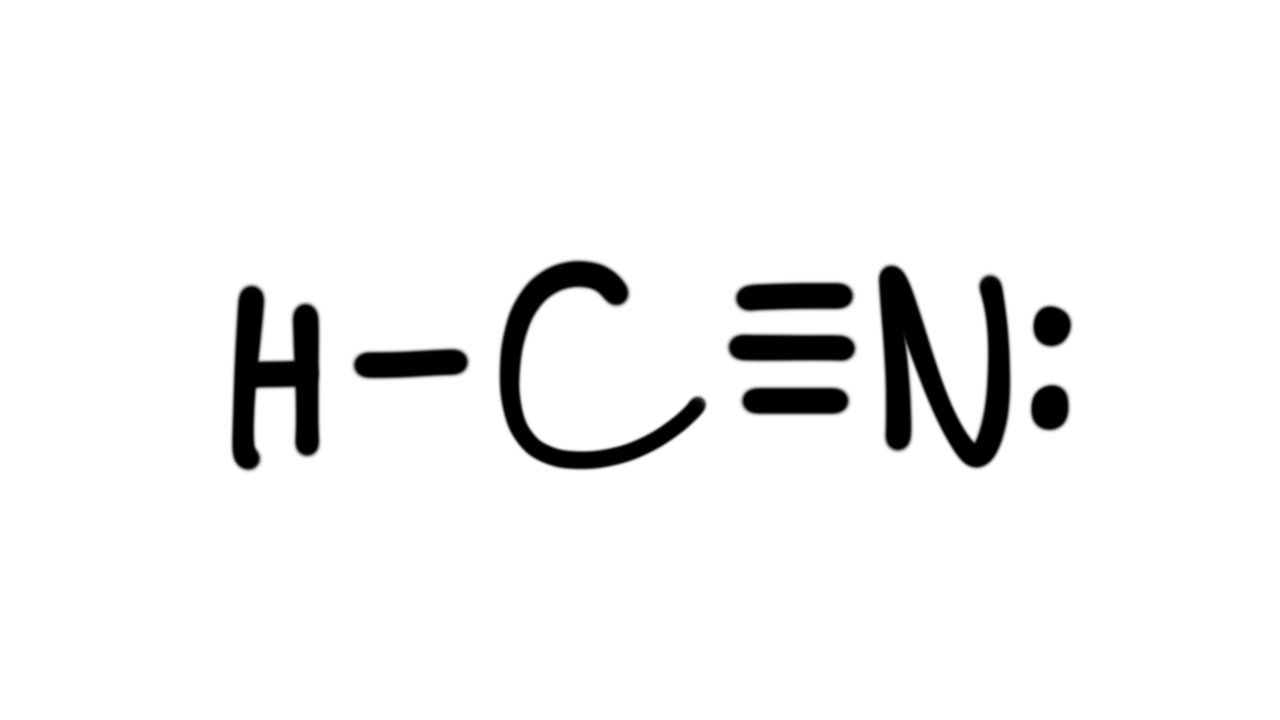Draw The Lewis Structure For The Hcn
Draw The Lewis Structure For The Hcn - Web draw out a correct lewis structure for the following compounds. Hcn consists of three atoms: Web unpaired electrons are called radicals, and you should avoid them. Now, use the information from step 4 and 5 to draw the lewis. Web hcn is a linear molecule, since it has only two electron groups and there are no lone pairs on the central carbon atom. Subtract step 3 number from step 1. Now we will draw the lewis dot structure of the compound. Web here's how to do it. (1 × × 1) + (4 × × 1. With the lewis structure for hcn you’ll need to share more than one pair of electrons between the carbon and the nitrogen atoms.
Web here's how to do it. Calculate the total number of valence electrons. Draw a skeleton structure put the least electronegative atom c in the middle with h and cl on either side. Now we will draw the lewis dot structure of the compound. Some compounds have a very unique and different lewis structure and hcn is one of those. All electrons should be in lone pairs or bonding pairs. Here, the given molecule is hcn. (1 × × 1) + (4 × × 1. Hcn, hydrogen cyanide, is a volatile and poisnous compound with distinguished bitter odor. Now, use the information from step 4 and 5 to draw the lewis.
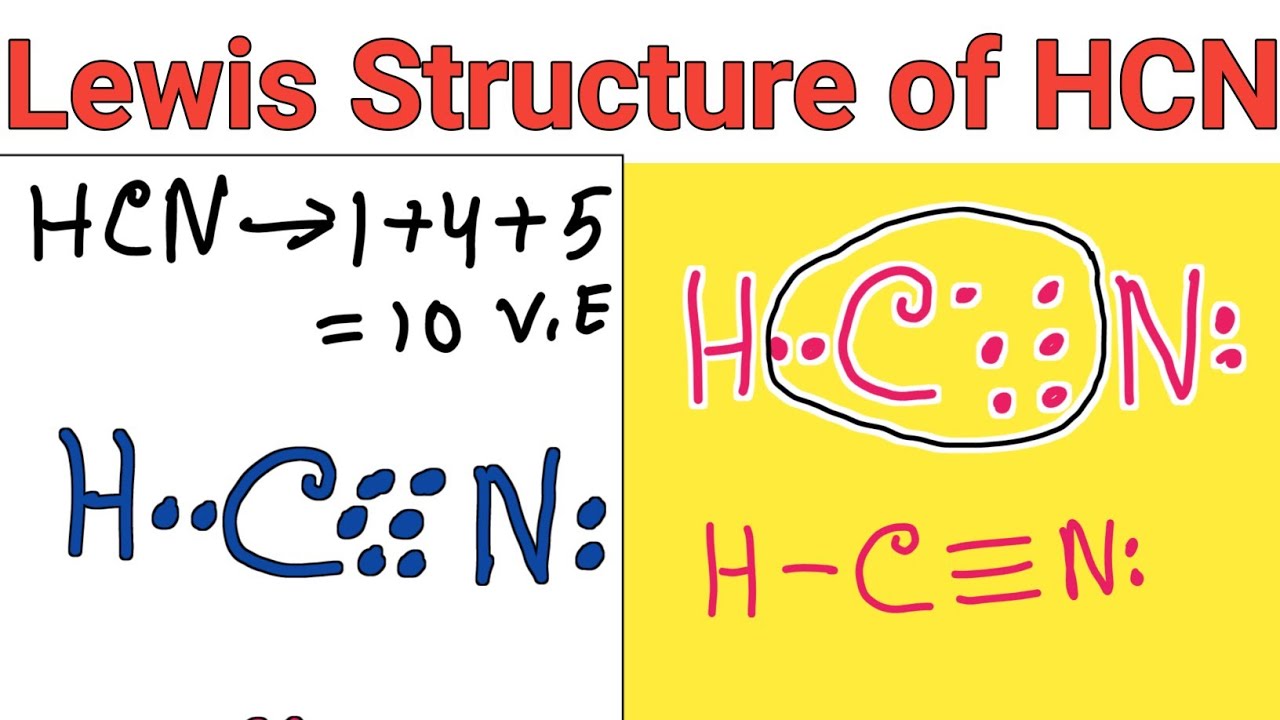
Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. Subtract step 3 number from step 1. (valence electrons are the number of electrons present in the outermost shell of an atom). Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for hydrogen cyanide (hcn). Make sure you put the correct atom at the center of the hcn molecule. (1 × × 1) + (4 × × 1. Web the lewis structure (lewis dot diagram) for hcn.1. Hcn, hydrogen cyanide, is a volatile and poisnous compound with distinguished bitter odor. Web here's how to do it. To know the valence electrons of hcn, let us go through the valence electrons of individual atoms in hydrogen cyanide.
Lewis Diagram For Hcn
This is crucial because valence electrons are the ones involved in chemical bonding. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Simplified lewis structure drawing for nonscience majors. journal of chemical education 75 (1998): Web how to draw the lewis dot structure of hydrogen cyanide (hcn) molecule in a simple and easy.
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
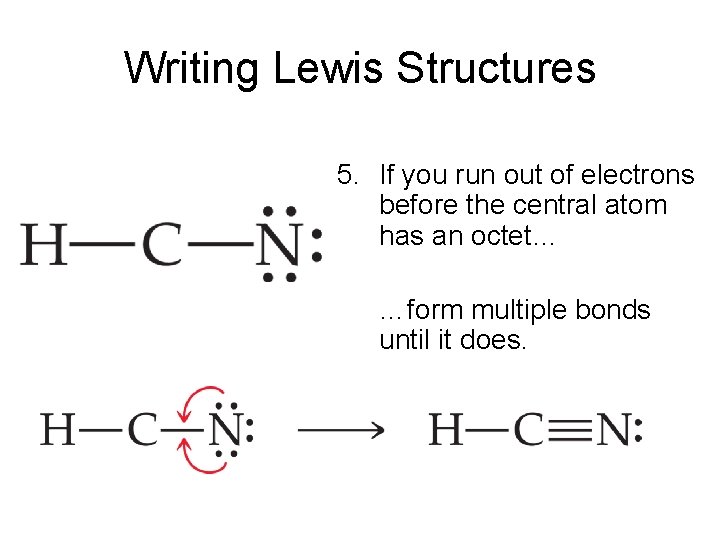
The hydrogen atom is bonded to the carbon atom, and the nitrogen atom is bonded to the carbon atom via a triple bond. Be sure that you don't use more than the ten valence electrons available. With the lewis structure for hcn you’ll need to share more than one pair of electrons between the carbon and the nitrogen atoms. 4.
Hcn Lewis Structure Bonds Draw Easy
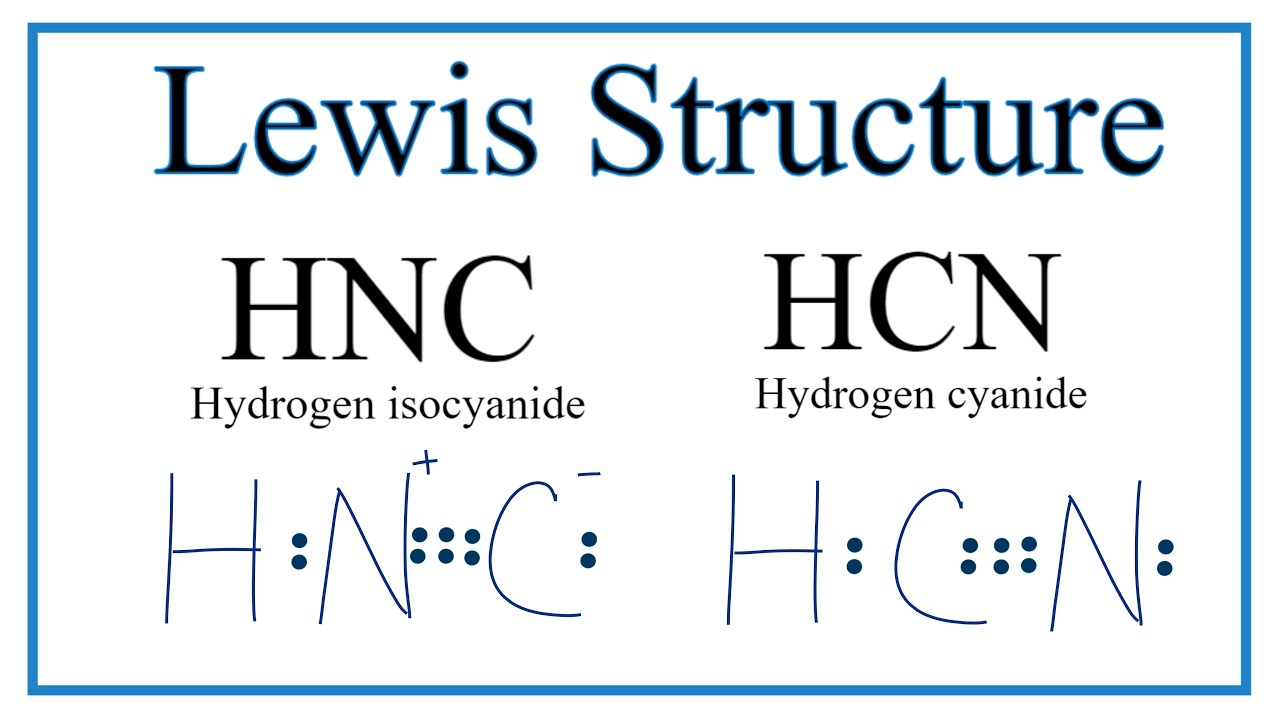
We'll also compare hnc to hcn and discuss why both are of inter. Web hcn is a linear molecule, since it has only two electron groups and there are no lone pairs on the central carbon atom. Subtract step 3 number from step 1. Hydrogen (h), carbon (c), and nitrogen (n). Hcn consists of three atoms:
Hcn Lewis Structure Bonds Draw Easy
Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Web here's how to do it. Web lewis structure of hcn. This is crucial because valence electrons are the ones involved in chemical bonding. 4 + (3 × 6) + 2 = 24 electrons.
HCN Lewis Structure (Hydrogen Cyanide) YouTube
Draw the lewis structure for hcn. Draw the lewis structure for the hcn molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web to draw the lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure. Step 2) attach the atoms to.
Lewis structure of HCN (Hydrogen cyanide) YouTube
Some compounds have a very unique and different lewis structure and hcn is one of those. Hcn consists of three atoms: All electrons should be in lone pairs or bonding pairs. Web here's how to do it. Web subtract step 1 total from step 2.
HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw
Web how to draw the lewis dot structure of hydrogen cyanide (hcn) molecule in a simple and easy way? Web draw out a correct lewis structure for the following compounds. Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for hydrogen cyanide (hcn). #1 first draw a rough sketch #2 mark lone pairs.
How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN
Draw the lewis structure for the hcn molecule. Hydrogen (h), carbon (c), and nitrogen (n). Draw the lewis structure for hcn. When you draw the lewis structure, make all the electrons paired unless there is an odd number of electrons. Web unpaired electrons are called radicals, and you should avoid them.
Hcn Lewis Structure Bonds Draw Easy
It is linear molecule with a triple bond between c and n atom and has bond angle of 180 degrees. Hcn, hydrogen cyanide, is a volatile and poisnous compound with distinguished bitter odor. Web draw out a correct lewis structure for the following compounds. Simplified lewis structure drawing for nonscience majors. journal of chemical education 75 (1998): #1 first draw.
Lewis Diagram For Hcn
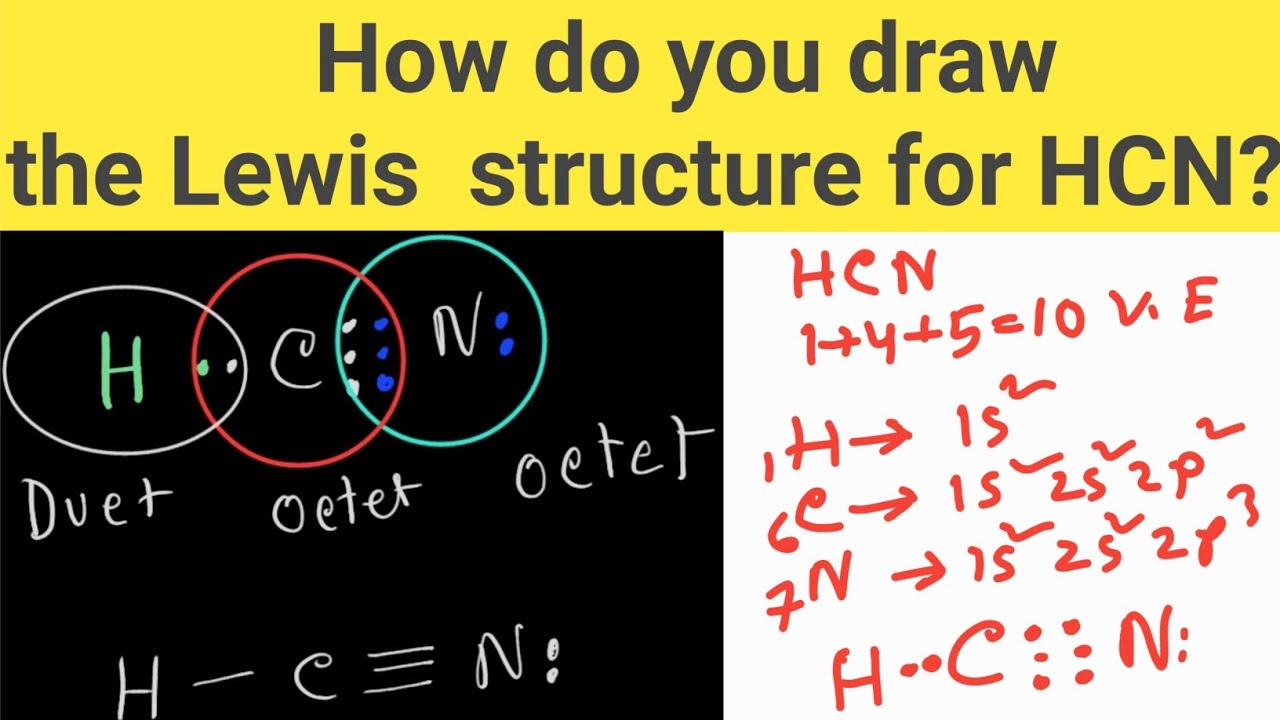
Web the hcn molecule consists of three atoms: Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Put one electron pair in each bond4. (1 × × 1) + (4 × × 1. Web unpaired electrons are called radicals, and.
Draw The Molecule By Placing Atoms On The Grid And Connecting Them With Bonds.
Put least electronegative atom in centre3. Hydrogen (h), carbon (c), and nitrogen (n). (1 × × 1) + (4 × × 1. Draw a skeleton structure put the least electronegative atom c in the middle with h and cl on either side.
Some Compounds Have A Very Unique And Different Lewis Structure And Hcn Is One Of Those.
To know the valence electrons of hcn, let us go through the valence electrons of individual atoms in hydrogen cyanide. Does this molecule exhibit resonance? Web the lewis structure (lewis dot diagram) for hcn.1. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets.
The Final Answer Must Have This Number Of Electrons‼!
The hydrogen atom is bonded to the carbon atom, and the nitrogen atom is bonded to the carbon atom via a triple bond. Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. Calculate the total number of valence electrons. Web here's how to do it.
The Sum Of The Valence Electrons Is 5 (From N) + 6 (From O) = 11.
Hydrogen (h), carbon (c), and nitrogen (n). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (there are molecules, like o 2, which have unpaired electrons even though they could all be paired, but you. Web draw out a correct lewis structure for the following compounds.