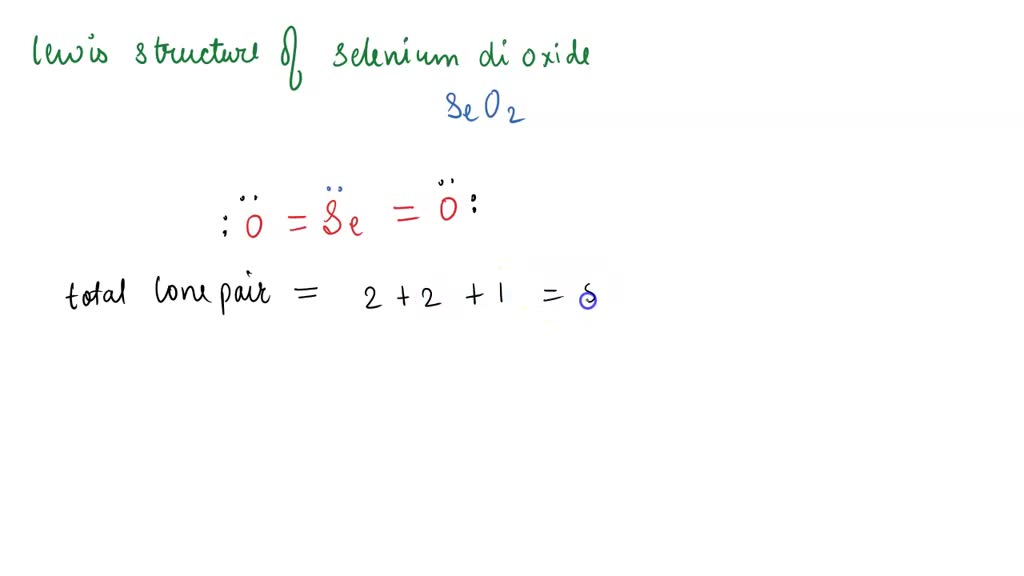Draw The Lewis Structure For The Selenium Dioxide
Draw The Lewis Structure For The Selenium Dioxide - Web seo 2 (selenium dioxide) has one selenium atom and two oxygen atoms. Web this problem has been solved! (valence electrons are the number of electrons present in the outermost shell of an atom). The lewis structure begins with determining the number of valence electrons already available with one selected molecule and how many are further needed to achieve a stable condition. In order to draw the lewis structure of seo2, first of all you have to find the total number of valence electrons present in the seo2 molecule. Selenium is the least electronegative and will act as the central atom. The two oxygen atoms are placed alongside it in the skeletal structure, as shown in the figure. Web this problem has been solved! Web hello guys!selenium dioxide is one of those molecules that can confuse you when it comes to determining its lewis structure. Here, the given molecule is seo2 (selenium dioxide).
Each oxygen atom has two lone pairs, and the selenium atom has one lone pair. (valence electrons are the number of electrons present in the outermost shell of an atom). Find more chemistry widgets in wolfram|alpha. Web hello guys!selenium dioxide is one of those molecules that can confuse you when it comes to determining its lewis structure. Here’s the best way to solve it. Web at this step of drawing the lewis structure of a molecule, we need to connect the outer atoms with the central atom using single straight lines. To draw the lewis structure of seo2, we need to follow a few steps:. Selenium is a nonmetal element with the atomic number 34 and the chemical symbol se. And we'll put two electrons between the atoms to form the bonds. Calculate the total number of valence electrons.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web at this step of drawing the lewis structure of a molecule, we need to connect the outer atoms with the central atom using single straight lines. С c r x o: Enter your answer in the provided box. Here, the given molecule is seo2 (selenium dioxide). Lewis dot structure with resonance. (b) select the correct vsepr geometry. Web steps to draw the lewis structure of selenium dioxide. What is the molecular shape, and the molecules overall polarity? Be sure to include all resonance structures that satisfy the octet rule.
Selenium Dioxide Lewis Structure
The two oxygen atoms are placed alongside it in the skeletal structure, as shown in the figure. To draw the lewis structure of seo2, we need to follow a few steps:. Se, on the periodic table, 6 valence electrons. In order to draw the lewis structure of seo2, first of all you have to find the total number of valence.
Selenium Dioxide Lewis Structure
Web this problem has been solved! Question 22 (15 points) listen draw the lewis structure of selenium dioxide (no need to post the picture). Although it is a linear molecule. Web steps to draw the lewis structure of selenium dioxide. Web the lewis structure for selenium dioxide (seo2) can be drawn following these steps:
[Solved] Draw the structural formula for selenium dioxide, Se O2, and
How many bonds are on the structure? In order to draw the lewis structure of seo2, first of all you have to find the total number of valence electrons present in the seo2 molecule. To draw the lewis structure of seo2, we need to follow a few steps:. (valence electrons are the number of electrons present in the outermost shell.
SeO2 Lewis Structure (Selenium Dioxide) Math, Lewis, Molecules
Calculate the total number of valence electrons. (valence electrons are the electrons that are. Enter your answer in the provided box. Draw the lewis dot diagram for selenium dioxide. Se, on the periodic table, 6 valence electrons.
Solved Draw the Lewis structure for the selenium dioxide
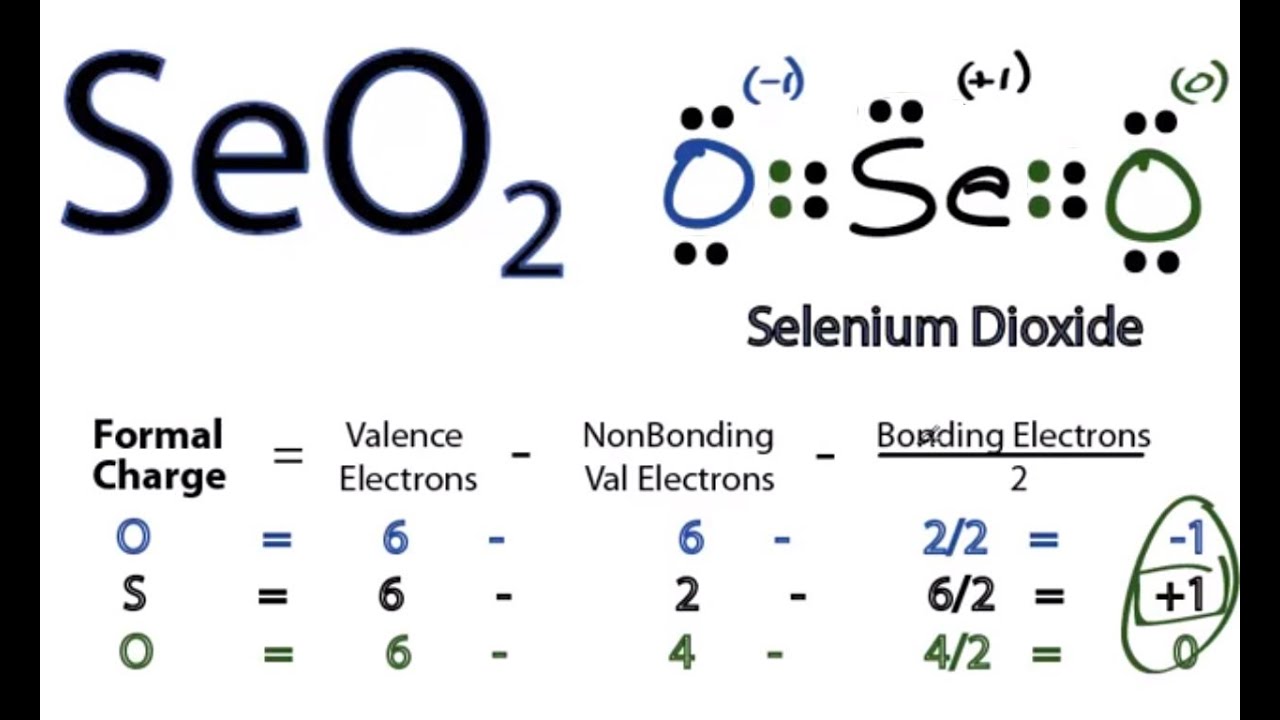
Be sure to include all resonance structures that satisfy the octet rule. Web let's do the seo2 lewis structure. Selenium is a nonmetal element with the atomic number 34 and the chemical symbol se. 6[se] + 12[o] = 18 valence electrons. The seo2 lewis structure represents the arrangement of atoms and valence electrons in a molecule of selenium dioxide (seo2).
Purchase Selenium dioxide [7446084] online • Catalog • Molekula Group
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web selenium dioxide is an oxide of selenium.it is used in organic synthesis, glass colorants, and as a toner in photographic developing. In seo2 there are 3 atoms sharing 2 bonds for which we need to assign the electrons in order to find the.
SOLVED draw the Lewis structure of selenium dioxide (no need to post a
The seo2 lewis structure represents the arrangement of atoms and valence electrons in a molecule of selenium dioxide (seo2). Draw the lewis dot diagram for selenium dioxide. (b) select the correct vsepr geometry. Tetrahedral o trigonal pyramidal angular linear trigonal planar 4. Find more chemistry widgets in wolfram|alpha.
SeO2 Lewis Structure How to Draw the Lewis Structure for SeO2 YouTube
Draw the lewis dot diagram for selenium dioxide. Oxygen 6, we've got two of them, for a total of 18 valence electrons. Make sure to account for the formal and/or ionic charge of each atom. Web this problem has been solved! And we'll put two electrons between the atoms to form the bonds.
Solved Draw the Lewis structure for the selenium dioxide
Web here are the steps i follow when drawing a lewis structure. Draw the lewis dot structure of the selenium dioxide (seo2).\table [ [\table [ [1) how many valence], [electrons in your compound?]],show calculation:,atanerathare], [2. Find the total valence electrons in seo2 molecule. Lewis dot structure with resonance. Web this problem has been solved!
Draw the Lewis structure for selenium dioxide YouTube
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Make sure to account for the formal and/or ionic charge of each atom. Each oxygen atom has two lone pairs, and the selenium atom has one lone pair. Calculate lewis dot structure for seo2. Enter your answer in the provided box.
Enter Your Answer In The Provided Box.
Find out valence electrons already present within one selenium dioxide. (valence electrons are the electrons that are. Web let's do the seo2 lewis structure. The seo2 lewis structure represents the arrangement of atoms and valence electrons in a molecule of selenium dioxide (seo2).
Web At This Step Of Drawing The Lewis Structure Of A Molecule, We Need To Connect The Outer Atoms With The Central Atom Using Single Straight Lines.
Determine the total number of valence electrons in seo2. Web this problem has been solved! 6[se] + 12[o] = 18 valence electrons. Here’s the best way to solve it.
Make Sure To Account For The Formal And/Or Ionic Charge Of Each Atom.
Selenium is the least electronegative and will act as the central atom. Be sure to answer all parts. How many bonds are on the structure? Se, on the periodic table, 6 valence electrons.
What Is The Molecular Shape, And The Molecules Overall Polarity?
Web steps to draw the lewis structure of selenium dioxide. Here, the given molecule is seo2 (selenium dioxide). Each oxygen atom has two lone pairs, and the selenium atom has one lone pair. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.



![Purchase Selenium dioxide [7446084] online • Catalog • Molekula Group](https://molekula.com/catalog/7446-08-4/30100275-selenium-dioxide/structure.png)