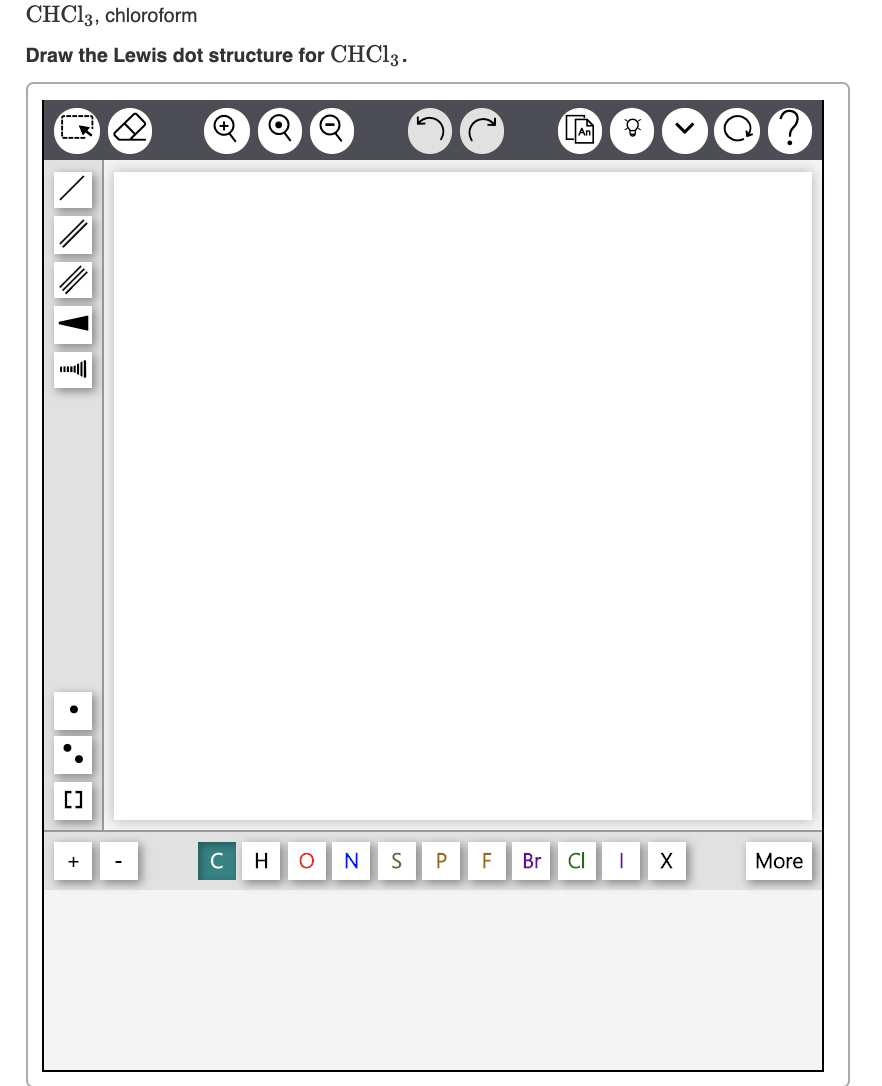
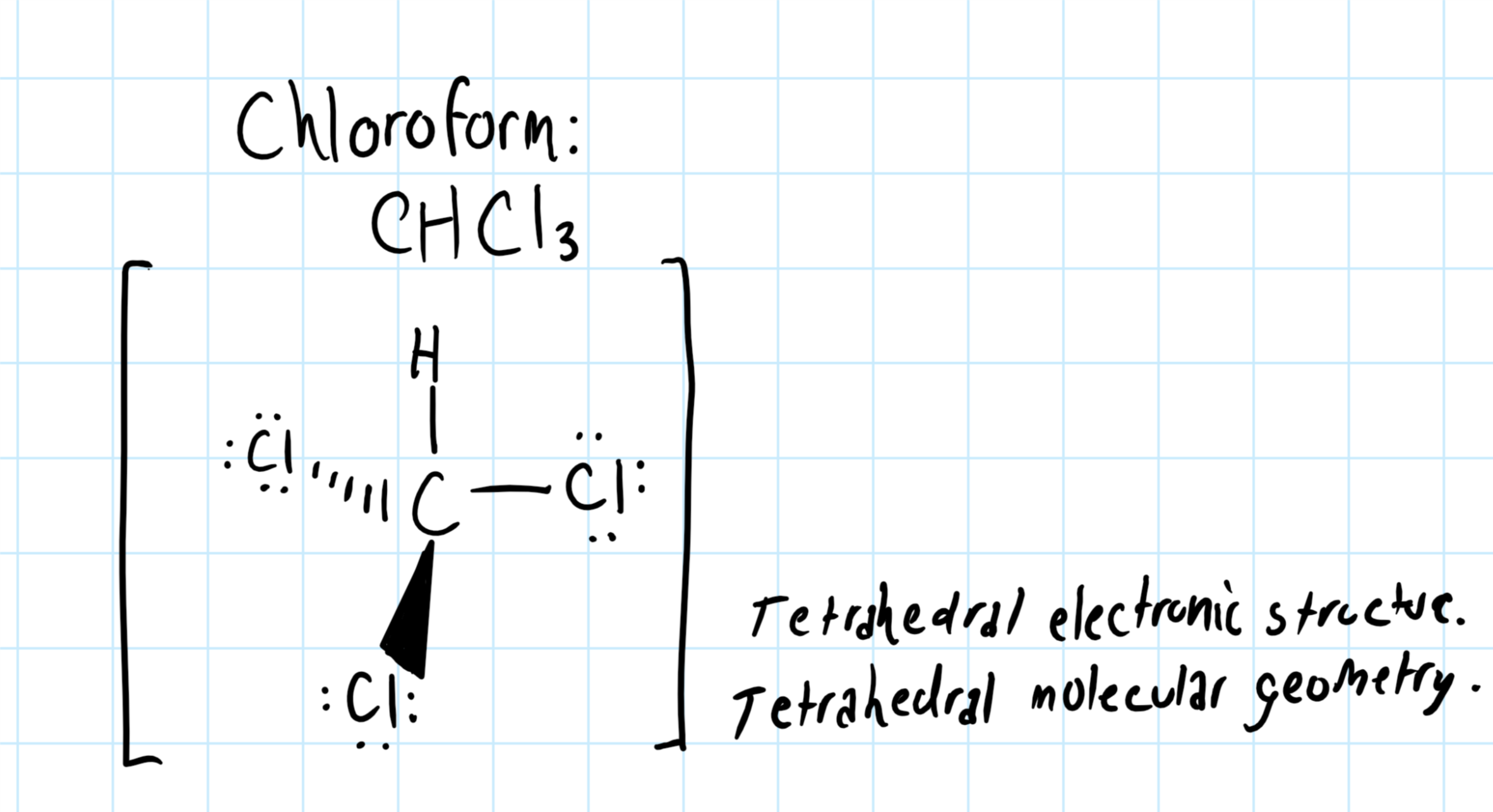
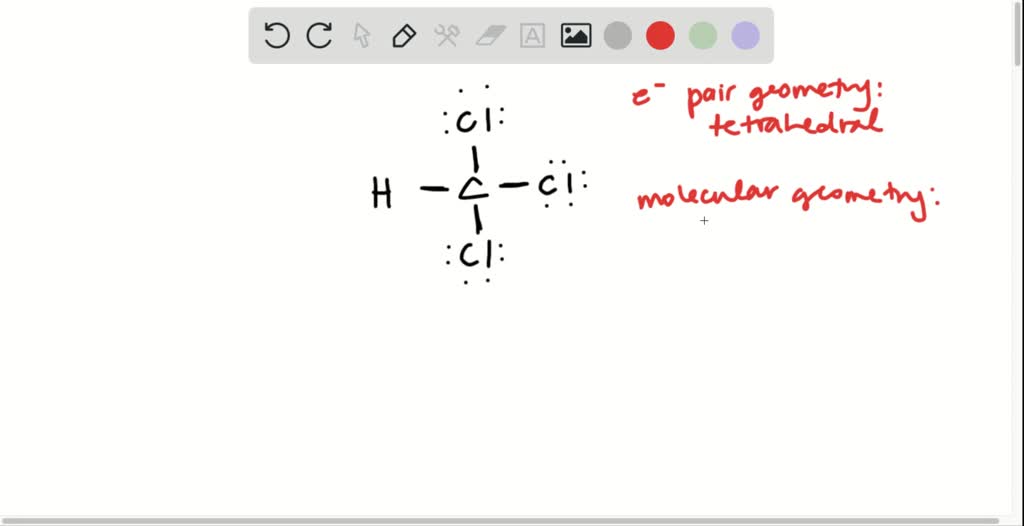
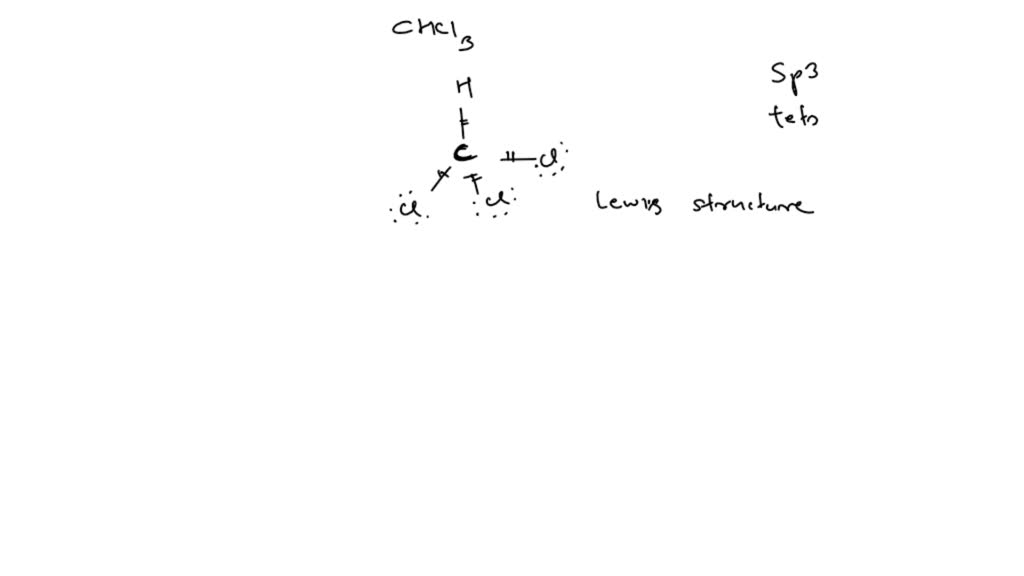
Draw The Lewis Structure Of Chloroform Chcl3
Draw The Lewis Structure Of Chloroform Chcl3 - #1 draw a rough sketch of the structure. Here, the given molecule is chcl3. 1) determine which atoms are connected to each other. It is also known as trichloromethane. This problem has been solved! For the chcl3 structure use the periodic table to find the total number of valence electrons for. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. The difference is that you have both cl and f. It is nonflammable and is denser than water. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom.
#2 next, indicate lone pairs on the atoms. 3) place two electrons between each atom in place of the bonds. Number of steps can be changed according the complexity of the molecule or ion. For the chcl 3 lewis structure there are a total of 26 valence electrons available. Web chloroform reacts with base sodium hydroxide forms sodium formate, sodium hydroxide and water. 100% (6 ratings) share share. #1 draw a rough sketch of the structure. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. The chemical equation is given below. Web drawing lewis structures for molecules with one central atom:
This problem has been solved! The chemical equation is given below. Remember that hydrogen (h) only needs 2 valence electrons for a full outer shell. Web it is obtained by subtracting the number of electrons assigned to an atom in the lewis structure from the number of valence electrons of the atoms in their free state. Chloroform is a clear, colorless liquid that possesses a pleasant odor. 2chcl3 + o2 → 2cocl2 + 2hcl. 1) determine which atoms are connected to each other. 3) place two electrons between each atom in place of the bonds. ) answer the following questions regarding chcl3 a) draw the lewis structure for the organic compound chloroform, chcl3 b) what is electronic geometry? It is nonflammable and is denser than water.
Solved CHCl3, chloroform Draw the Lewis dot structure for
3) place two electrons between each atom in place of the bonds. 4) add the rest of the available valence electrons to complete the octet of the surrounding atoms. Find the total valence electrons in chcl3 molecule. Draw the lewis structure of chloroform, chcl3. However those all steps are mentioned and explained in detail in this tutorial for your knowledge.
CHCl3 Lewis Structure How to Draw the Lewis Structure for CHCl3 YouTube
The difference is that you have both cl and f. The chemical equation is given below. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Chloroform is a clear, colorless liquid that possesses a pleasant odor. 2chcl3 + o2 → 2cocl2 + 2hcl.
CHCl3 Molecular Geometry / Shape and Bond Angles (Chloroform) YouTube
Chcl3 lewis structure, molecular geometry, hybridization, bond angle and shape. 2) determine the number of valence electrons in the molecule. #1 draw a rough sketch of the structure. Web a lewis structure for chcl3.(chem 1090 lewis 3b) This organic compound consists of one carbo.more.
CHCl3Lewisstructure Chloroform Chloroformlewisstructure
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure of chloroform, also called trichloromethane, (chcl3) and then determine the ideal bonding angle (s) of the central atom. The lewis structure for chcl 3 is similar to cf 4 or ccl 4. Here’s the best way to solve it. Chcl3.
SOLVED Draw the Lewis structure for chloroform, CHCl3. What are its
4) add the rest of the available valence electrons to complete the octet of the surrounding atoms. Web lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each.
How would you draw the chloroform molecule (CHCl_3)? Socratic
Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. C) what is molecular geometry? In order to draw the lewis structure of chcl3, first of all you have to find the total.
Draw the Lewis structure for chloroform, CHCl3 What are its electron
2) determine the number of valence electrons in the molecule. (valence electrons are the number of electrons present in the outermost shell of an atom). This problem has been solved! Here’s the best way to solve it. Chloroform on oxidation forms phosgene and hydrochloric acid.
Draw the Lewis structure for chloroform, CHCl _{3}.… SolvedLib
Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. For chcl3, we have 1 carbon atom with 4 valence electrons and 3 chlorine atoms with 7 valence electrons each, giving a total of 26 valence electrons. It is nonflammable and is denser than water. When we draw a lewis structure,.
SOLVEDValence Bond Theory Draw the Lewis structure for chloroform
Web draw the lewis structure of chloroform, chcl3. Web steps of drawing lewis structure of chcl 3. Here’s the best way to solve it. (valence electrons are the number of electrons present in the outermost shell of an atom). Remember that hydrogen (h) only needs 2 valence electrons for a full outer shell.
CHCl3 Lewis Structure, Geometry, Hybridization, and Polarity
The chemical equation is given below. Here, the given molecule is chcl3. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. D) what is bond angles? 4) add the rest of the available valence electrons to complete the octet of the surrounding atoms.
4) Add The Rest Of The Available Valence Electrons To Complete The Octet Of The Surrounding Atoms.
Number of steps can be changed according the complexity of the molecule or ion. The chemical equation is given below. It is nonflammable and is denser than water. Draw the lewis structure of chloroform, chcl3.
The Following Procedure Can Be Used To Draw Lewis Structure For Simple Molecules.
3) place two electrons between each atom in place of the bonds. Web a lewis structure for chcl3.(chem 1090 lewis 3b) 2) determine the number of valence electrons in the molecule. Chloroform on oxidation forms phosgene and hydrochloric acid.
#1 Draw A Rough Sketch Of The Structure.
This problem has been solved! Web here are the steps to draw the chcl3 lewis structure: Web steps of drawing lewis structure of chcl 3. Web it is obtained by subtracting the number of electrons assigned to an atom in the lewis structure from the number of valence electrons of the atoms in their free state.
Hello Guys!Chcl3 Is A Chemical.
Chloroform, also called trichloromethane, (chcl3) is an important laboratory solvent with a relatively high vapor pressure at room temperature. For the chcl 3 lewis structure there are a total of 26 valence electrons available. It is calculated by adding up the valence electrons on all atoms. Here’s the best way to solve it.