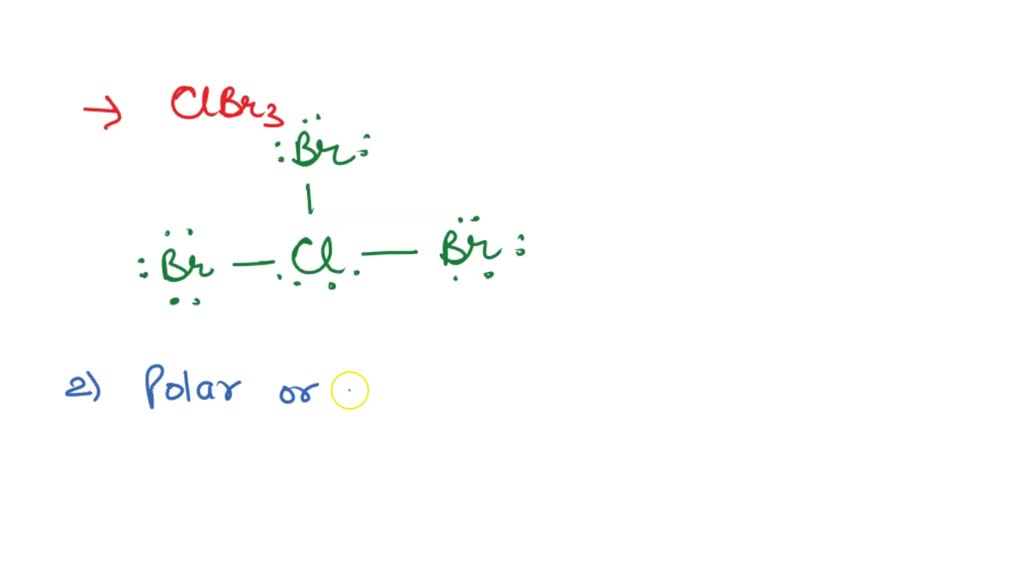Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs
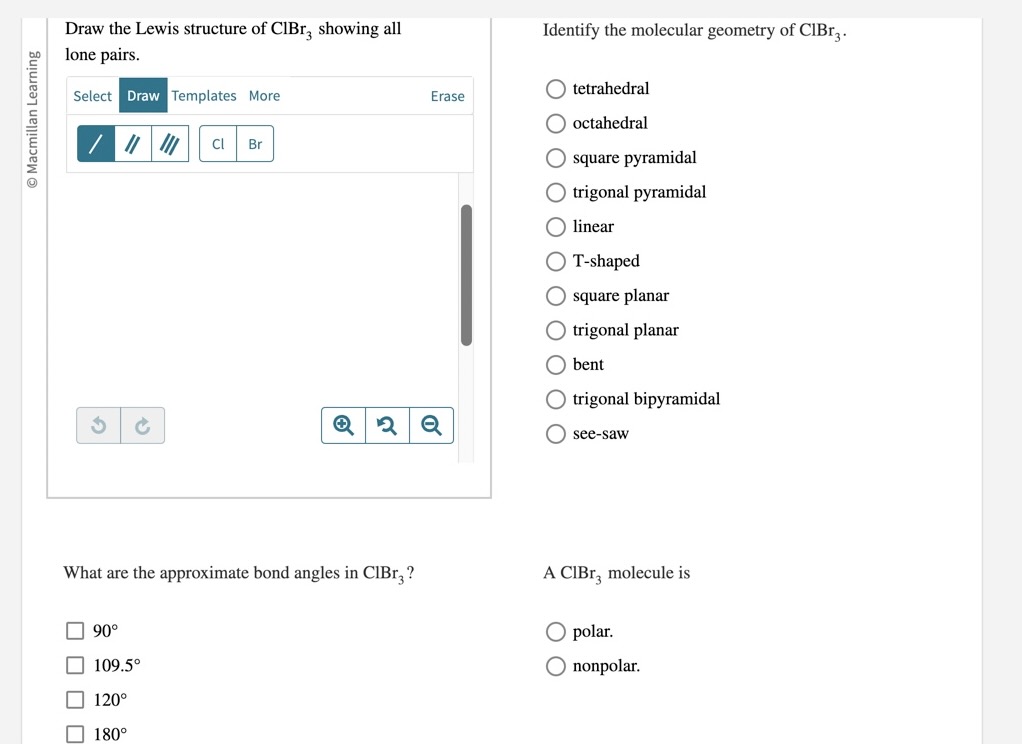
Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs - The chlorine atom has 2 lone pairs and all the three bromine atoms have 3 lone pairs. Identify the molecular geometry of cibr_3. Web clbr3 lewis structure has an chlorine atom (cl) at the center which is surrounded by three bromine atoms (br). Web lewis structure of clbr3 contains three single bonds between the chlorine (cl) atom and each bromine (br) atom. What are the approximate bond angles in clbr3? Each cl atom interacts with eight valence electrons: Lewis structures involve the chemical symbols of the elements which take part in chemical bonding. O trigonal pyramidal square pyramidal linear what are the approximate bond angles in. Two are in the first energy level, and seven are in the second level. By signing up, you accept quizlet's terms of service and privacy policy.
Web this widget gets the lewis structure of chemical compounds. Cl have two lone pairs. Identify the molecular geometry of clbr3. In the lewis structure of clbr3, each chlorine bonds with three bromine atoms. Identify the molecular geometry of clbr3. O trigonal pyramidal square pyramidal linear what are the approximate bond angles in. This problem has been solved! They can also be called lewis dot diagrams and are used as a simple way to show the configuration of atoms within a molecule. Web clbr3 lewis structure has an chlorine atom (cl) at the center which is surrounded by three bromine atoms (br). #3 indicate formal charges on the atoms, if necessary.
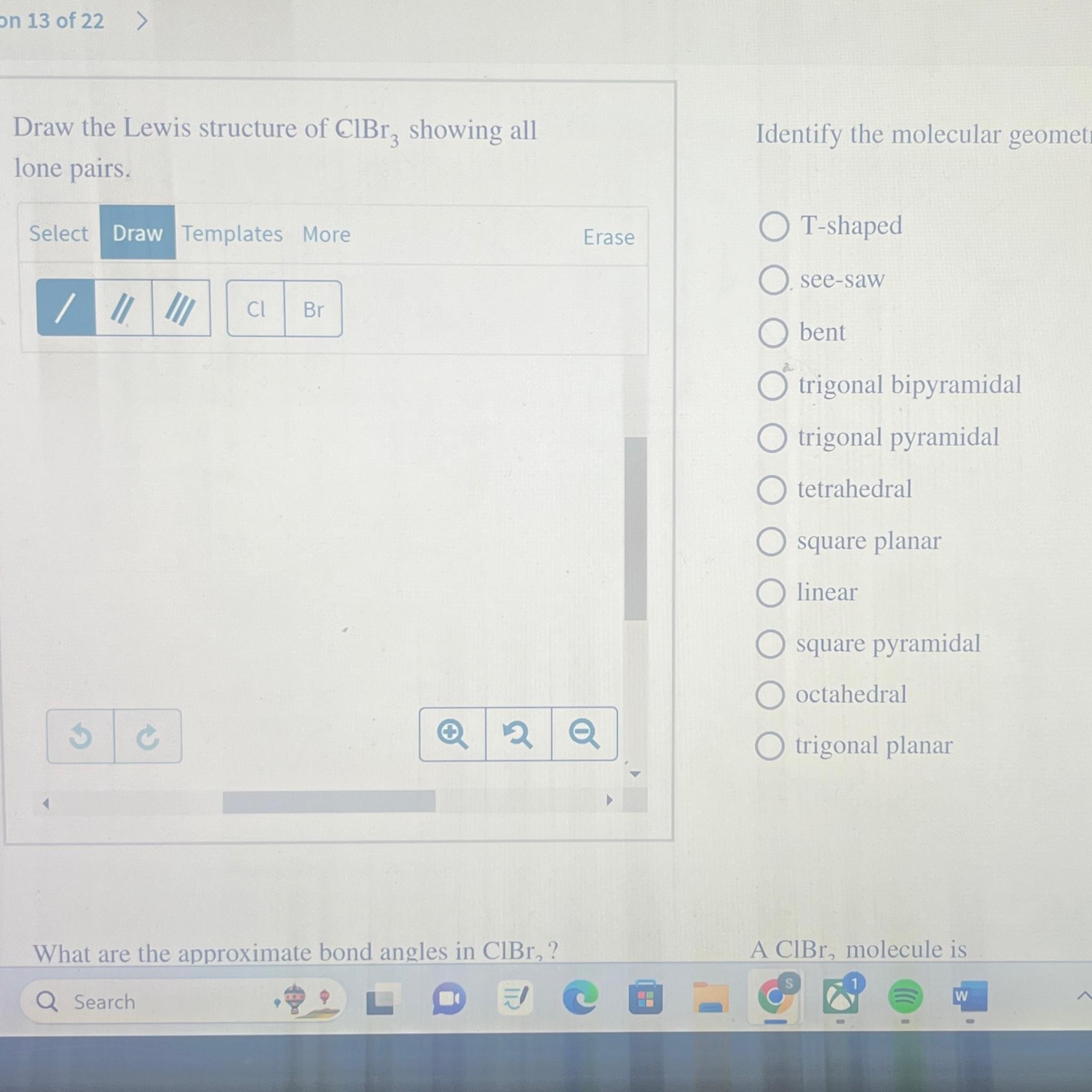
Octahedral see‑saw tetrahedral bent square pyramidal t‑shaped square planar trigonal planar linear trigonal bipyramidal trigonal pyramidal what are the approximate bond angles in clbr3? #2 next, indicate lone pairs on the atoms. #3 indicate formal charges on the atoms, if necessary. Web draw the lewis structure of clbr3 showing all lone pairs. Cl have two lone pairs. There are 2 lone pairs on the chlorine atom (cl) and 3 lone pairs on all three bromine atoms (br). The lewis structure for , likely a typo for , consists of a central iodine atom bonded to three chlorine atoms, each with three lone pairs. There are 3 single bonds between the chlorine atom (cl) and each bromine atom (br). The chlorine atom (cl) is at the center and it is surrounded by 3 bromine atoms (br). A cibr_3 molecule is polar.
OneClass Draw the lewis structure of ClBr3 showing all lone pairs.
#1 draw a rough sketch of the structure. This helps us figure out how to show their electrons in the lewis structure. A cibr_3 molecule is polar. #3 indicate formal charges on the atoms, if necessary. Create a free account to view solutions.
Solved Draw the Lewis structure of ClBr3 showing all Inna
Let’s draw and understand this lewis dot structure. Web draw the lewis structure of clbr3 showing all lone pairs. The lewis structure for , likely a typo for , consists of a central iodine atom bonded to three chlorine atoms, each with three lone pairs. #3 indicate formal charges on the atoms, if necessary. Octahedral see‑saw tetrahedral bent square pyramidal.
Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs 55+ Pages
There are 2 lone pairs on the chlorine atom (cl) and 3 lone pairs on all three bromine atoms (br). What are the approximate bond angles in clbr3? There are 3 single bonds between the chlorine atom (cl) and each bromine atom (br). Web draw the lewis structure for {eq}clbr_3 {/eq} and determine its electron geometry and molecular geometry. Draw.
Solved on 13 of 22>Draw the Lewis structure of ClBr3
Draw the lewis structure of clbr3 showing all lone pairs. Identify the molecular geometry of cibr_3. A cibr3 molecule is polar. Identify the molecular geometry of clbr3. There are 3 single bonds between the chlorine atom (cl) and each bromine atom (br).
How To Draw Lewis Structures A Step By Step Tutorial
Identify the molecular geometry of clbr3. What are the approximate bond angles in clbr3? The lewis structure of clbr3 includes central atom bromine (br) sharing one electron pair with each of three chlorine atoms (cl). #2 next, indicate lone pairs on the atoms. Lewis structures involve the chemical symbols of the elements which take part in chemical bonding.
Solved 1. Follow the steps for drawing Lewis structures and
Let’s draw and understand this lewis dot structure. They can also be called lewis dot diagrams and are used as a simple way to show the configuration of atoms within a molecule. The chlorine atom (cl) is at the center and it is surrounded by 3 bromine atoms (br). #1 draw a rough sketch of the structure. Identify the molecular.
[Solved] Draw the Lewis structure of ClBr3 with lone pairs. Course Hero
#1 draw a rough sketch of the structure. All the br atoms are octet fulfilled. Chlorine tribromide is a polar molecule due to presence of electronegativity difference between chlorine and bromine atoms and it is an interhalogen compound. Br 109.5° 120° 180° : The lewis structure of clbr3 includes central atom bromine (br) sharing one electron pair with each of.
OneClass Draw complete Lewis structure, including lone pairs, for the
#1 draw a rough sketch of the structure. Web this widget gets the lewis structure of chemical compounds. This helps us figure out how to show their electrons in the lewis structure. By signing up, you accept quizlet's terms of service and privacy policy. Central atom have total 10 electrons.
SOLVED 'Draw the lewis structure of ClBr3 showing all lone pairs.
Chlorine tribromide is a polar molecule due to presence of electronegativity difference between chlorine and bromine atoms and it is an interhalogen compound. A cibr_3 molecule is polar. Draw the lewis structure of clbr3 showing all lone pairs. Identify the molecular geometry of cibr_3. Lewis structures involve the chemical symbols of the elements which take part in chemical bonding.
SOLVED Draw the Lewis structure for PF, . including lone pairs. Select
All the br atoms are octet fulfilled. The chlorine atom (cl) is at the center and it is surrounded by 3 bromine atoms (br). #3 indicate formal charges on the atoms, if necessary. Each cl atom interacts with eight valence electrons: Cl have two lone pairs.
Web Draw The Lewis Structure For {Eq}Clbr_3 {/Eq} And Determine Its Electron Geometry And Molecular Geometry.
Draw the lewis structure of cibr_3 showing all lone pairs. All the br atoms are octet fulfilled. Draw the lewis structure of clbr3 showing all lone pairs. Draw the lewis structure of cibr₃, showing all lone pairs.
Web Clbr3 Lewis Structure Has An Chlorine Atom (Cl) At The Center Which Is Surrounded By Three Bromine Atoms (Br).
A cibr_3 molecule is polar. Central atom cl which have excess electron than octet. #1 draw a rough sketch of the structure. Octahedral see‑saw tetrahedral bent square pyramidal t‑shaped square planar trigonal planar linear trigonal bipyramidal trigonal pyramidal what are the approximate bond angles in clbr3?
Web A Lewis Structure Is A Representation Of Covalent Molecules (Or Polyatomic Ions) Where All The Valence Electrons Are Shown Distributed About The Bonded Atoms As Either Shared Electron Pairs (Bond Pairs) Or Unshared Electron Pairs (Lone Pairs).
Web lewis structure of clbr3 contains three single bonds between the chlorine (cl) atom and each bromine (br) atom. Each cl atom interacts with eight valence electrons: Web to properly draw the clbr 3 lewis structure, follow these steps: What are the approximate bond angles in clbr3?
Lewis Dot Structure Of Clbr3.
Web this widget gets the lewis structure of chemical compounds. Lewis structures show all of the valence electrons in an atom or molecule. Identify the molecular geometry of clbr3. This helps us figure out how to show their electrons in the lewis structure.