Draw The Lewis Structure Of Ph3
Draw The Lewis Structure Of Ph3 - Coming to the contribution of. Drawing the lewis structure for ph 3. Count the valence electrons in your trial structure (8). Draw the lewis structure of ph3. B) draw a lewis structure for so42∗ in which all atoms obey the octet rule. In the structure of phosphane, we can see that there are 3 atoms of hydrogen element one phosphorus element atom is present. Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. To properly draw the ph 3 lewis structure, follow these steps: Phosphorus atom is in the centre forming single bonds with three hydrogen atoms and also has a lone pair of electrons in its lewis structure. Web chemistry questions and answers.
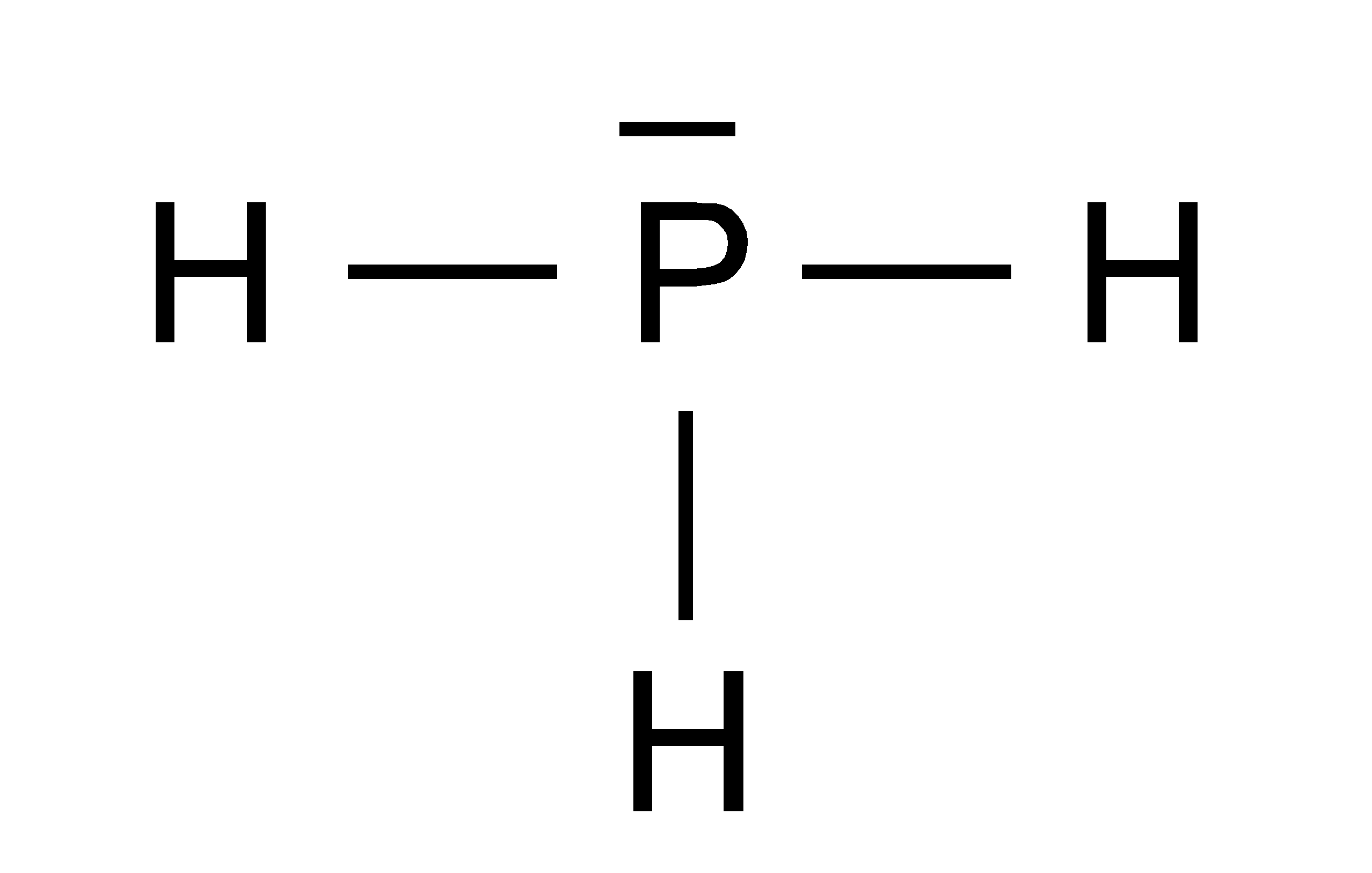
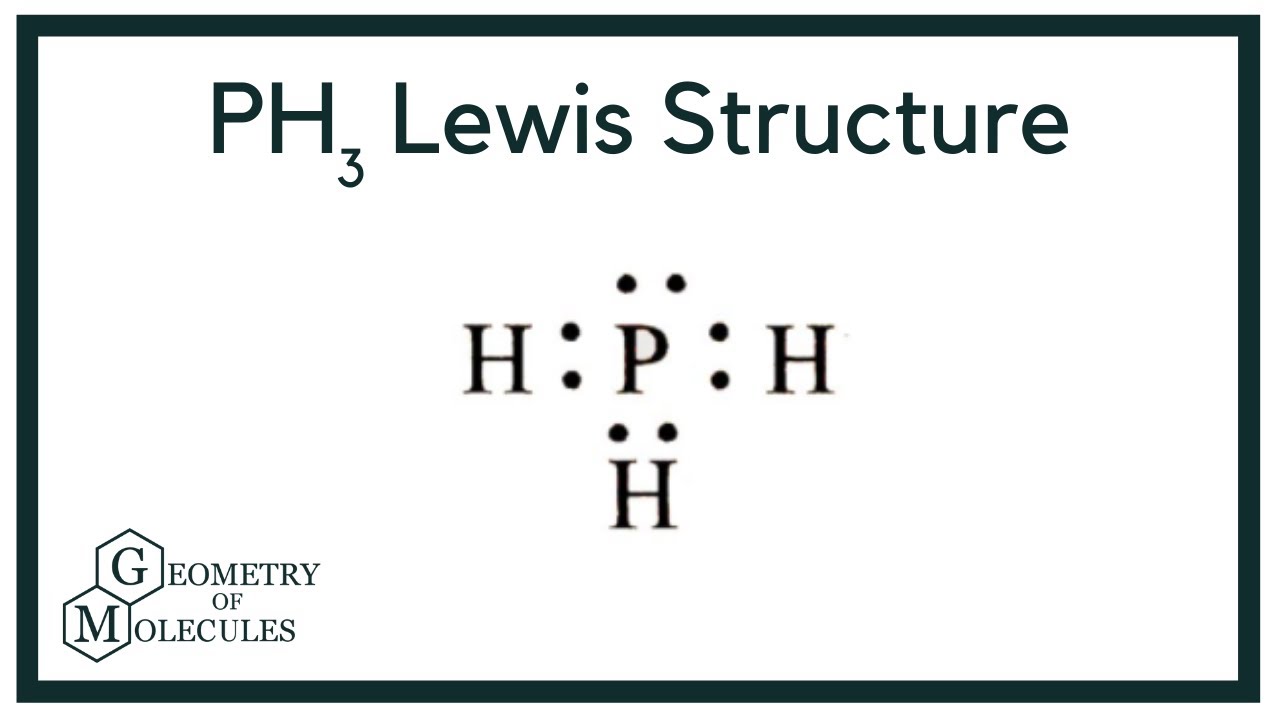
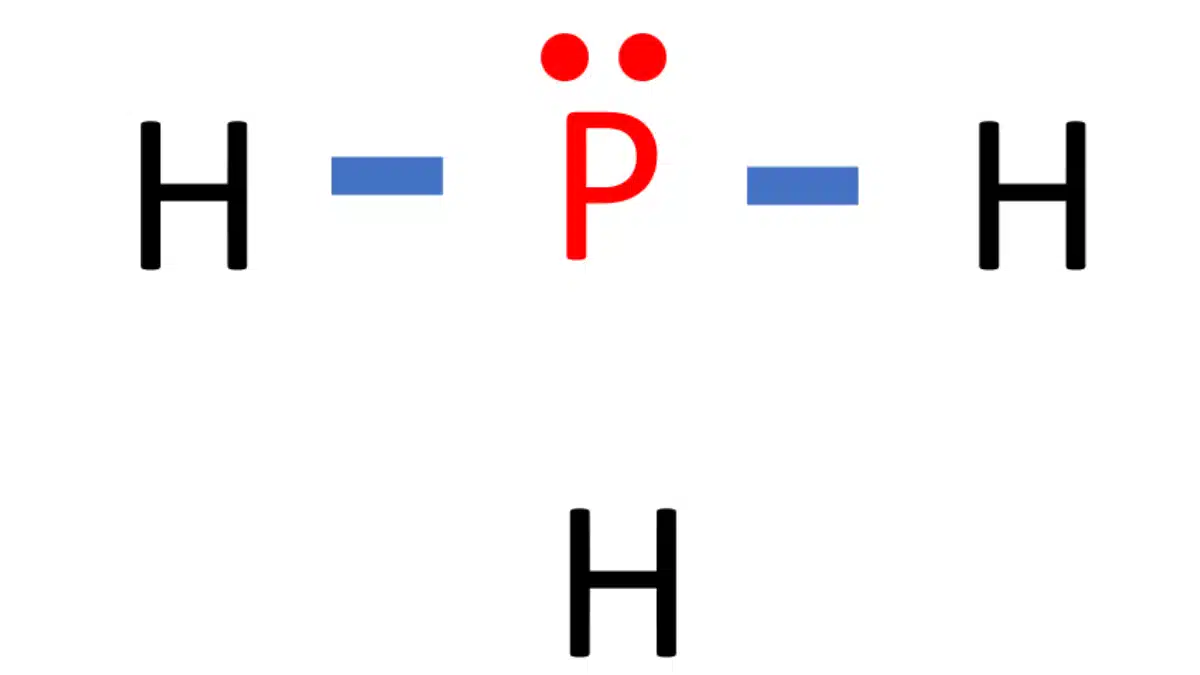
Draw the lewis structure of ph3. Web placing one bonding pair of electrons between the o atom and each h atom gives h:o:h, with 4 electrons left over. #3 indicate formal charges on the atoms, if necessary. In order to draw the lewis structure of ph3, first of all you have to find the total number of valence electrons present in the ph3 molecule. This is the lewis structure we drew earlier. Here, the given molecule is ph3. Web there are eight valence electrons for the ph3 molecule. Those steps are explained in detail in this tutorial. Web steps of drawing lewis structure of ph 3. For ph3, this sums up to 8 valence electrons.
Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. To properly draw the ph 3 lewis structure, follow these steps: The trial structure has exactly the same number of electrons as we have available. But, because phosphine is a simple molecule, these steps are not complex and do not require all general steps which are used to draw lewis structures of complex molecules and ions. This is the lewis structure we drew earlier. Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Since hydrogen is in group i it has one (1) valence electron in. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Hydrogen has an electron configuration of 1s^1. Welcome back to geometry of molecules where we make chemistry fun and easy.
Draw the Lewis dot structure for PH3 YouTube
Web the lewis structure for ph 3 is similar to nh 3. This problem has been solved! Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. For the ph3 lewis structure we first count the valence electrons for the ph3 molecule using the periodic table. Coming to the contribution of.
Ph3 Lewis Structure Shape
Web this type of lewis dot structure is represented by an atomic symbol and a series of dots. In the structure of phosphane, we can see that there are 3 atoms of hydrogen element one phosphorus element atom is present. #1 draw a rough sketch of the structure. Hydrogen has an electron configuration of 1s^1. Part a draw the lewis.
PH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle and
Web 6 steps to draw the lewis structure of ph3 step #1: Coming to the contribution of. Web chemistry questions and answers. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. Hydrogen has an electron configuration of 1s^1.
Draw The Lewis Structure Of Ph3
Web how to draw lewis structure for ph3? To write the ph3 lewis structure one should know the total of all the valence electrons that could be present in the molecule of ph3. Draw the lewis structure of ph3. Welcome back to geometry of molecules where we make chemistry fun and easy. This is the lewis structure we drew earlier.
PH3 Lewis Structure (Phosphine) YouTube
This problem has been solved! Web how to draw lewis structure for ph3? There are several steps to draw the lewis structure of ph 3. Now, count the valence electrons you actually have available. Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule.
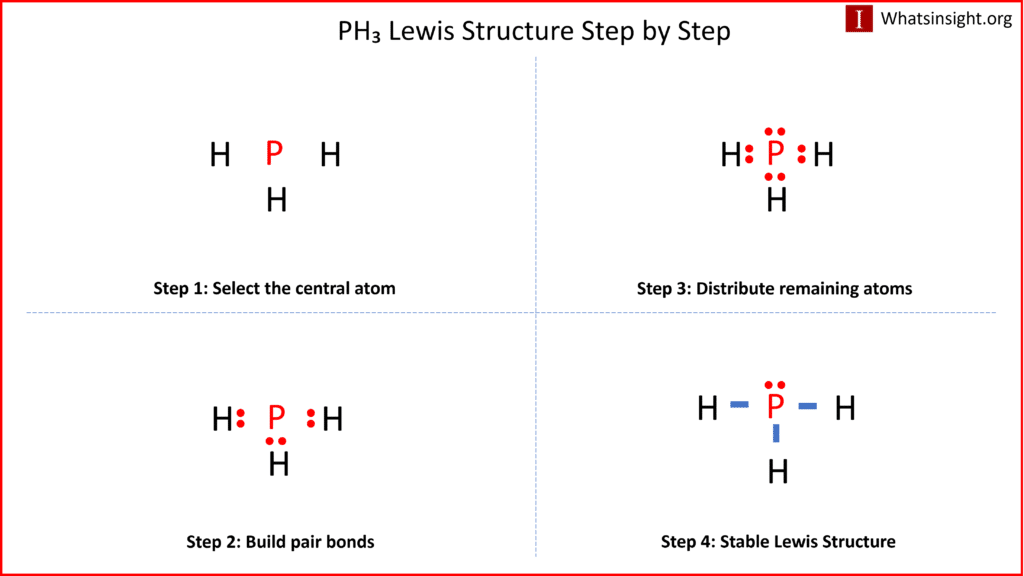
PH3 Lewis Structure in four simple steps What's Insight
Web the partial lewis structure that follows is for a hydrocarbon molecule. In order to draw the lewis structure of ph3, first of all you have to find the total number of valence electrons present in the ph3 molecule. In the full lewis structure, each carbon atom satisfies the octet rule, and there are no unshared electron pairs in the.
PH3 Lewis Structure in four simple steps What's Insight
To properly draw the ph 3 lewis structure, follow these steps: This problem has been solved! Web draw the lewis structure for ph3 (p is the central atom) and answer the questions below. Once we know how many valence electrons there are in ph3 we can distribute them around the central atom and attempt to fill the outer shells of.
Ph3 Lewis Structure Shape
Each h atom has a full valence shell of 2 electrons. Web the partial lewis structure that follows is for a hydrocarbon molecule. Hydrogen has an electron configuration of 1s^1. Web chemistry questions and answers. The molecular geometry and shape of the ph3 molecule is a trigonal pyramid.
Draw The Lewis Structure Of Ph3 Drawing.rjuuc.edu.np
Coming to the contribution of. Web the lewis structure for ph 3 is similar to nh 3. A) draw a lewis structure for ph3 in which all atoms except hydrogen obey the octet rule. Calculate the total number of valence electrons. Web 6 steps to draw the lewis structure of ph3 step #1:
PH3 Lewis Structure How to Draw the Lewis Structure for PH3 YouTube
A) draw a lewis structure for ph3 in which all atoms except hydrogen obey the octet rule. Draw the lewis dot structure for the hydrogen atom. (valence electrons are the number of electrons present in the. For ph3, this sums up to 8 valence electrons. In the structure of phosphane, we can see that there are 3 atoms of hydrogen.
Three Pairs Will Be Used In The Chemical Bonds.
Web the lewis structure for ph 3 is similar to nh 3. Web placing one bonding pair of electrons between the o atom and each h atom gives h:o:h, with 4 electrons left over. 4 + (3 × 6) + 2 = 24 electrons. Web chemistry questions and answers.
In Order To Draw The Lewis Structure Of Ph3, First Of All You Have To Find The Total Number Of Valence Electrons Present In The Ph3 Molecule.
Draw the lewis structure of ph3. During the bonding process, phosphorous is surrounded by three hydrogen atoms, and each is connected by a single bond. But, because phosphine is a simple molecule, these steps are not complex and do not require all general steps which are used to draw lewis structures of complex molecules and ions. The two remaining electrons form a lone pair.
#1 Draw A Rough Sketch Of The Structure.
Part a draw the lewis structure of ph3 to add lone pairs, click the button before clicking on the molecule. The carbon—carbon bonds are labeled 1, 2, and 3. Calculate the total number of valence electrons. 1 p + 3 h =.
In The Structure Of Phosphane, We Can See That There Are 3 Atoms Of Hydrogen Element One Phosphorus Element Atom Is Present.
#3 indicate formal charges on the atoms, if necessary. Each of the three hydrogens will fill one. Phosphosus has an electron configuration of [ne] 2s^2 2p^3. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.