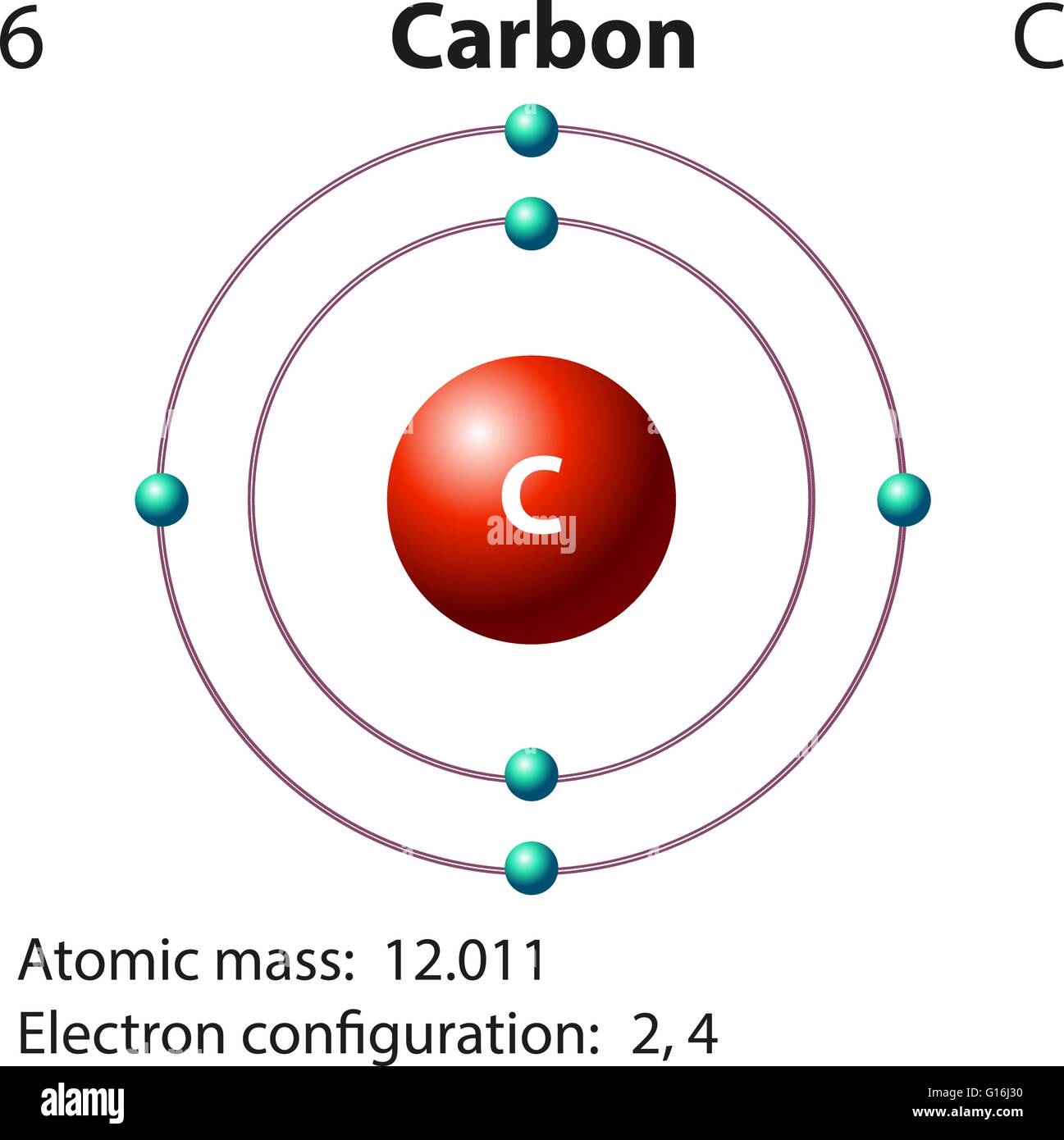
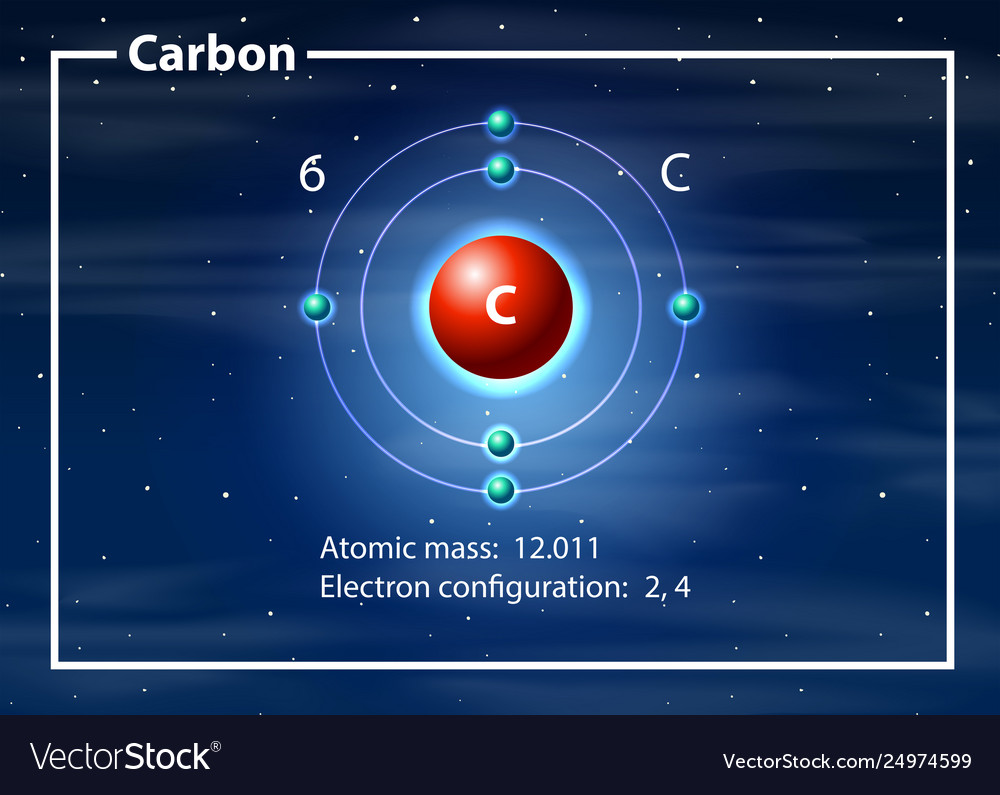
Drawing Of Carbon Atom
Drawing Of Carbon Atom - Rule 1 carbon atoms aren’t usually shown. Web the atomic number for carbon is 6, so you'll need 6 protons, and in turn 6 electrons. Hydrogen atoms are omitted but are assumed to be present to complete each of carbon's four bonds. Occasionally, a carbon atom might be indicated for emphasis or clarity. Carbon typically shares electrons to achieve a complete valence shell, forming bonds with multiple other atoms. Web the rules for drawing skeletal structures are straightforward. Carbon has 2 electrons in its first. Rule 2 hydrogen atoms bonded to carbon aren’t shown. Hold down the alt key to modify standard angles. Occasionally, a carbon atom might be indicated for emphasis or clarity.
56k views 4 years ago. Now it is possible for some of the elements of the second period to not have eight electrons. Web choose from carbon atom drawing stock illustrations from istock. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Hydrogens that are attached to elements other than carbon are shown. The circle with the c is a representative nucleus, so now you'll need to indicate the electron orbitals. Web a carbon atom is present wherever a line intersects another line. Explain the importance of carbon skeleton structures. Carbon typically shares electrons to achieve a complete valence shell, forming bonds with multiple other atoms. Hydrogen atoms bonded to carbon aren’t shown.
Rule 1 carbon atoms aren’t usually shown. Hydrogen atoms bonded to carbon aren’t shown. A picture of a hydrogen atom can be found here. Web carbon (c ), as a group 14 element, has four electrons in its outer shell. Hydrogen atoms are omitted but are assumed to be present to complete each of carbon's four bonds. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Now it is possible for some of the elements of the second period to not have eight electrons. Web these are therefore two isotopes of carbon. Rule 1 carbon atoms aren’t usually shown. The number of rings you need is to tied to the number of electrons you have.
Carbon Atom Ascension Glossary
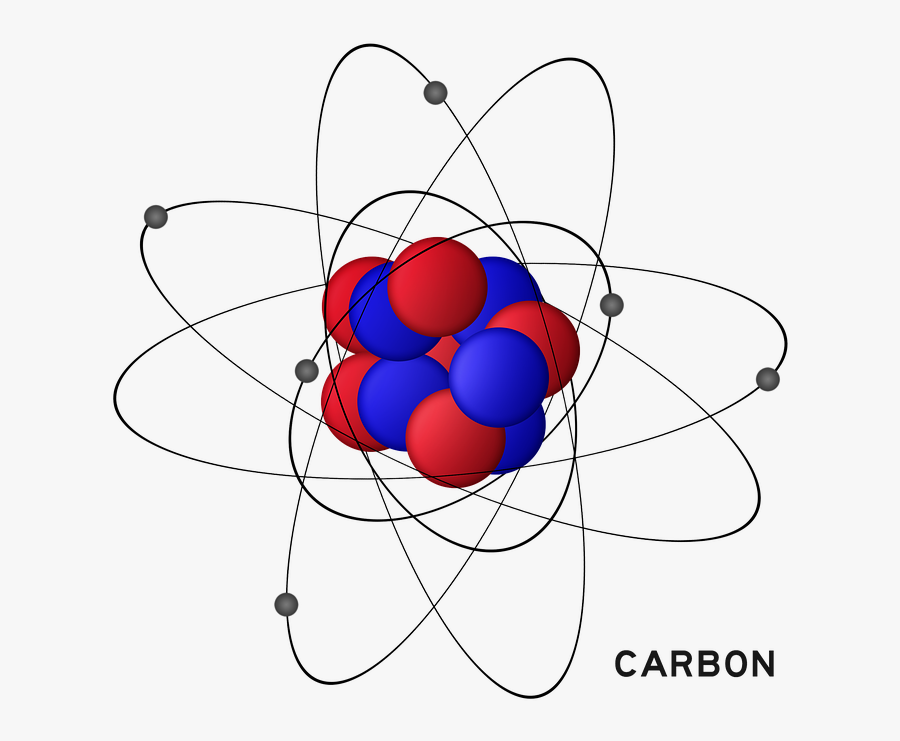
[/caption]the image on the left is a basic atom diagram. Rule 2 hydrogen atoms bonded to carbon aren’t shown. Occasionally, a carbon atom might be indicated for emphasis or clarity. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Hydrogen atoms bonded to carbon aren’t shown.
Carbon atom Royalty Free Vector Image VectorStock
Web explore carbon structure, understand what a carbon skeleton is, how to draw it, and what it looks like. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. The purple numbers indicate how much. Hold down the alt key to modify standard angles. Now it is possible.
Drawing Atoms Montessori Muddle
Into carbon monoxide (one oxygen atom). Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web february 17, 2010 by jerry coffey. Web carbon (c ), as a group 14 element, has four electrons in its outer shell. Molview consists of two main.
Carbon atomic structure (437243) Illustrations Design Bundles
Web the atomic number for carbon is 6, so you'll need 6 protons, and in turn 6 electrons. Web draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. [/caption]the image on the left is a basic atom diagram. Web we will use this information to draw the bohr model of.
Carbon Atom Diagram
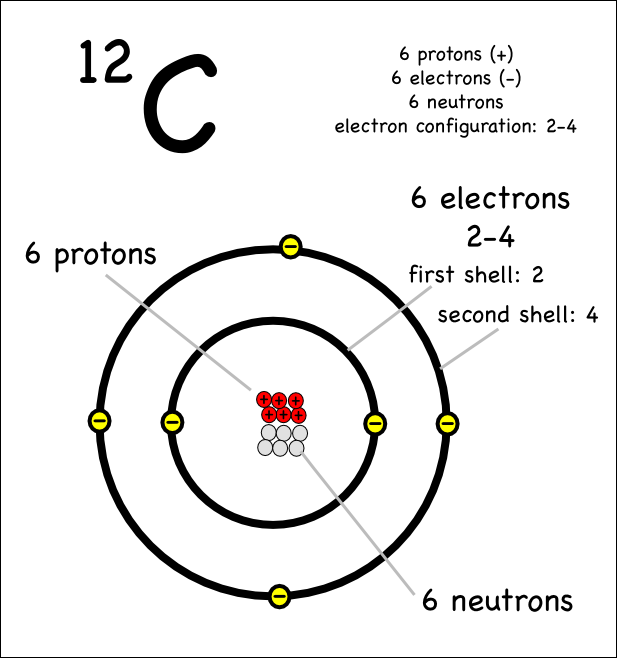
The chain size is displayed at the end of the chain. Web these are therefore two isotopes of carbon. Web we will use this information to draw the bohr model of the carbon atom. For this, we will first have to calculate the number of protons and neutrons present in this atom. The structural formula editor is surround by three.
3d render of atom structure of carbon isolated over white background
Web february 17, 2010 by jerry coffey. Web carbon (c ), as a group 14 element, has four electrons in its outer shell. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Occasionally, a carbon atom might be indicated for emphasis or clarity. Hold down the.
Carbon atom diagram hires stock photography and images Alamy
Web carbon (c ), as a group 14 element, has four electrons in its outer shell. Rule 1 carbon atoms aren’t usually shown. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Occasionally, a carbon atom might be indicated for emphasis or clarity. Web diagram of.
REMC 22 / Figure App
Molview consists of two main parts, a structural formula editor and a 3d model viewer. Rule 2 hydrogen atoms bonded to carbon aren’t shown. A picture of a hydrogen atom can be found here. Into carbon monoxide (one oxygen atom). Web these are therefore two isotopes of carbon.
Carbon atom diagram hires stock photography and images Alamy
Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Also, helium is shown in group 8a, but it only has two valence electrons. Web choose from carbon atom drawing stock illustrations from istock. The circle with the c is a representative nucleus, so now you'll need to.
Carbon atom diagram concept Royalty Free Vector Image
Rule 2 hydrogen atoms bonded to carbon aren’t shown. Hydrogen atoms bonded to carbon aren’t shown. Web explore carbon structure, understand what a carbon skeleton is, how to draw it, and what it looks like. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons..
56K Views 4 Years Ago.
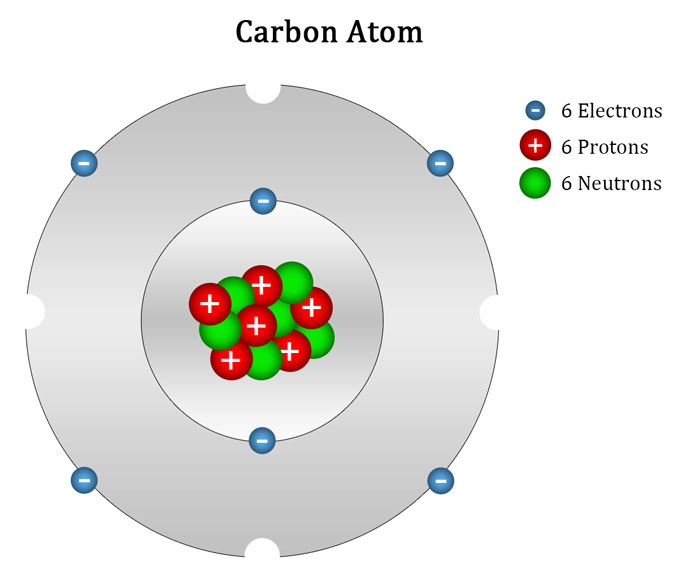
Carbon typically shares electrons to achieve a complete valence shell, forming bonds with multiple other atoms. Hold down the alt key to modify standard angles. The purple numbers indicate how much. Web the atomic number for carbon is 6, so you'll need 6 protons, and in turn 6 electrons.
Also, Helium Is Shown In Group 8A, But It Only Has Two Valence Electrons.
Web a more appropriate sanction would draw on trump’s history of adopting highways and attaching a sign thanking himself for beautifying them. Web draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. A picture of a hydrogen atom can be found here. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then.
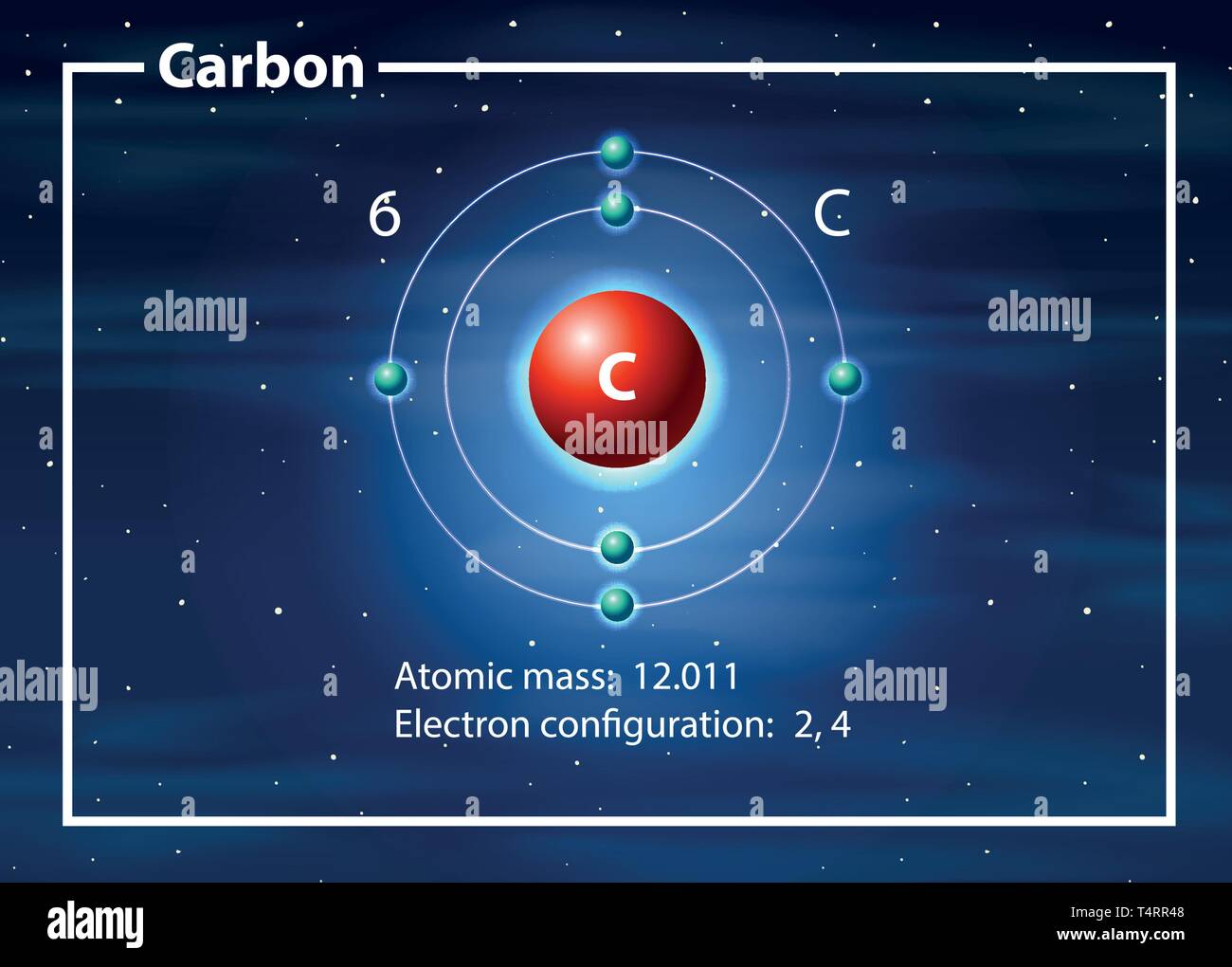
Carbon Has 2 Electrons In Its First.
Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. The black numbers indicate how much carbon is stored in various reservoirs, in billions tonnes (gtc stands for gigatonnes of carbon; Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Occasionally, a carbon atom might be indicated for emphasis or clarity.
Hydrogen Atoms Bonded To Carbon Aren’t Shown.
Rule 1 carbon atoms aren’t usually shown. Web diagram of the carbon cycle. Web february 17, 2010 by jerry coffey. For this, we will first have to calculate the number of protons and neutrons present in this atom.