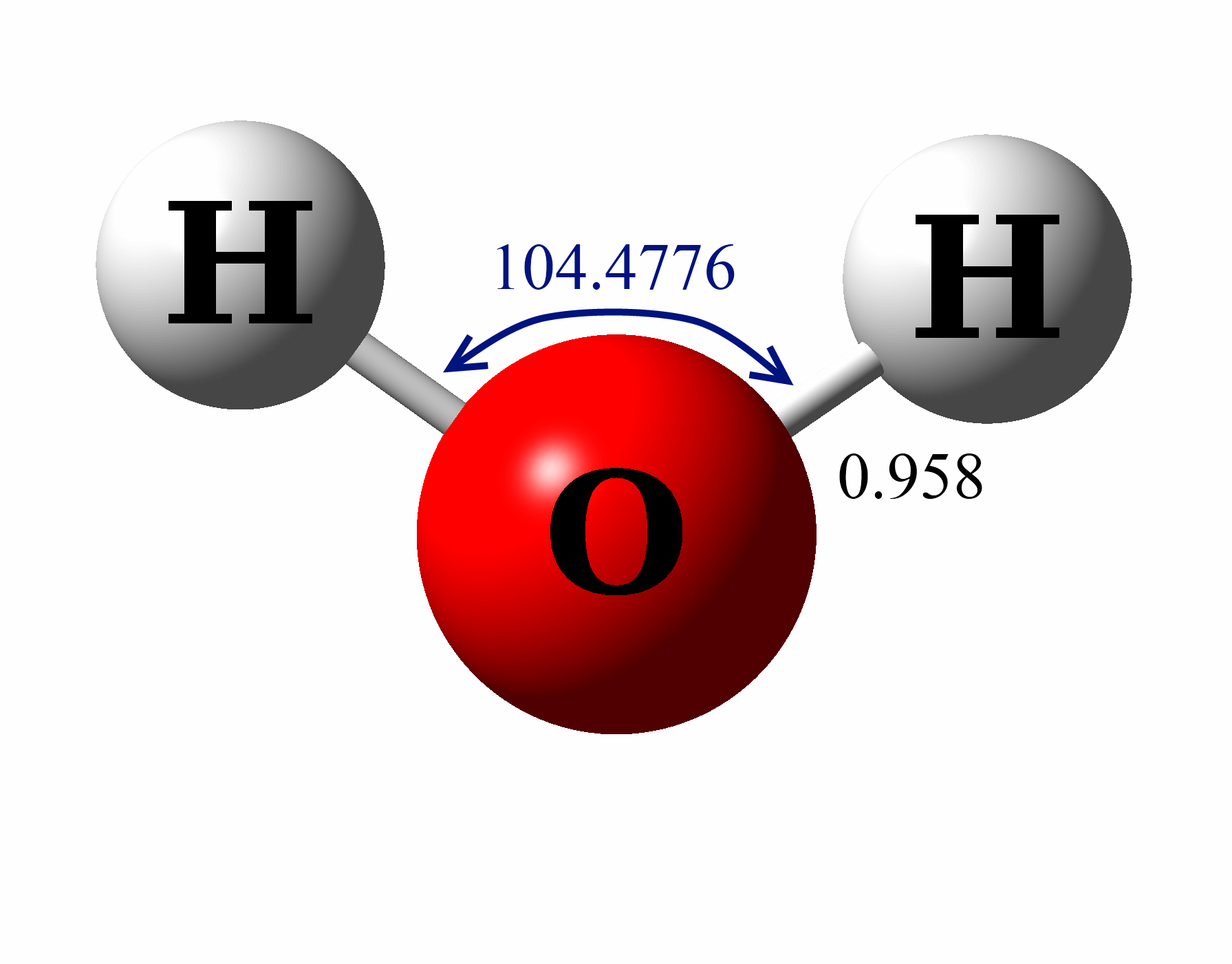
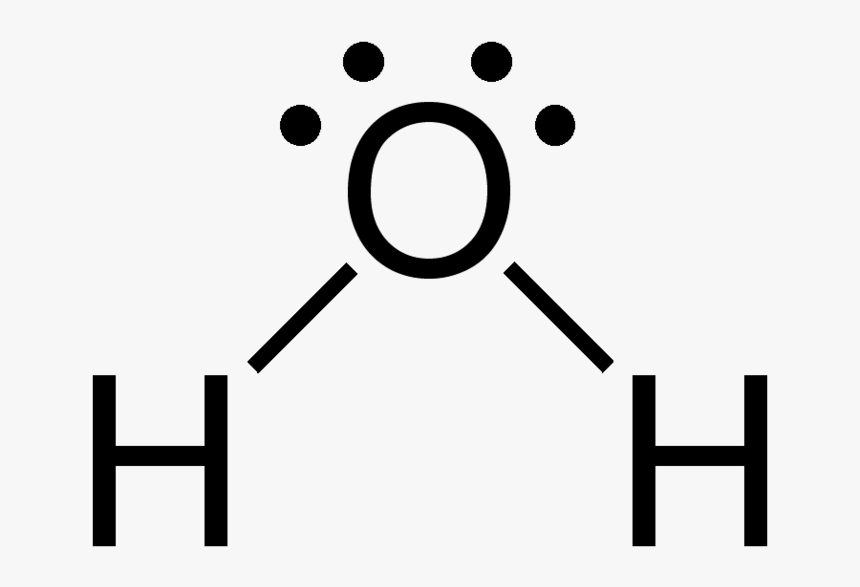
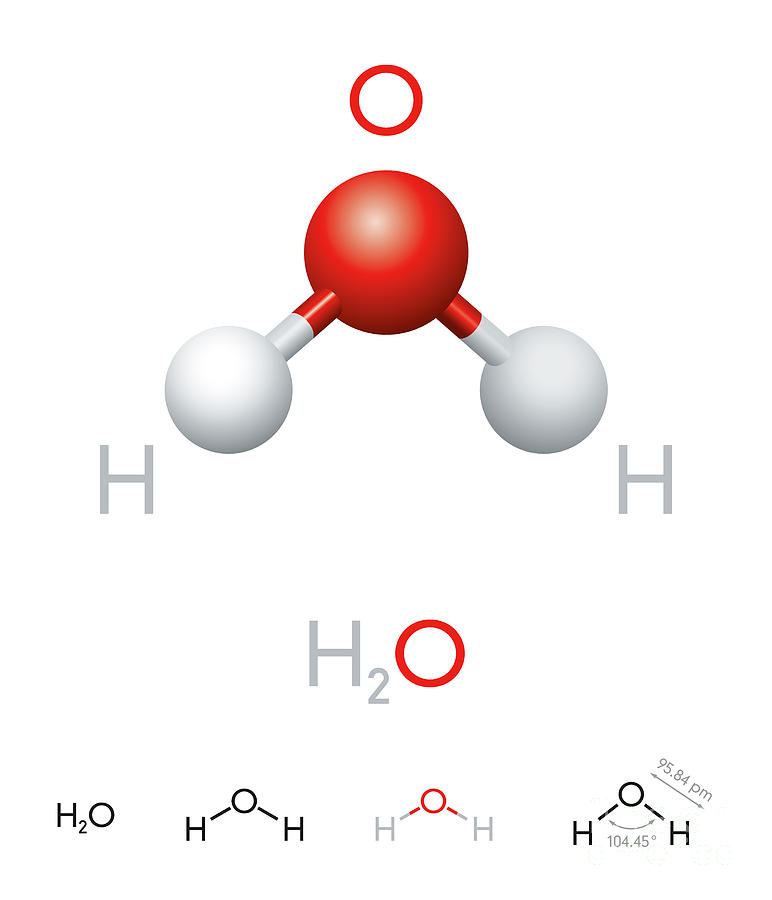
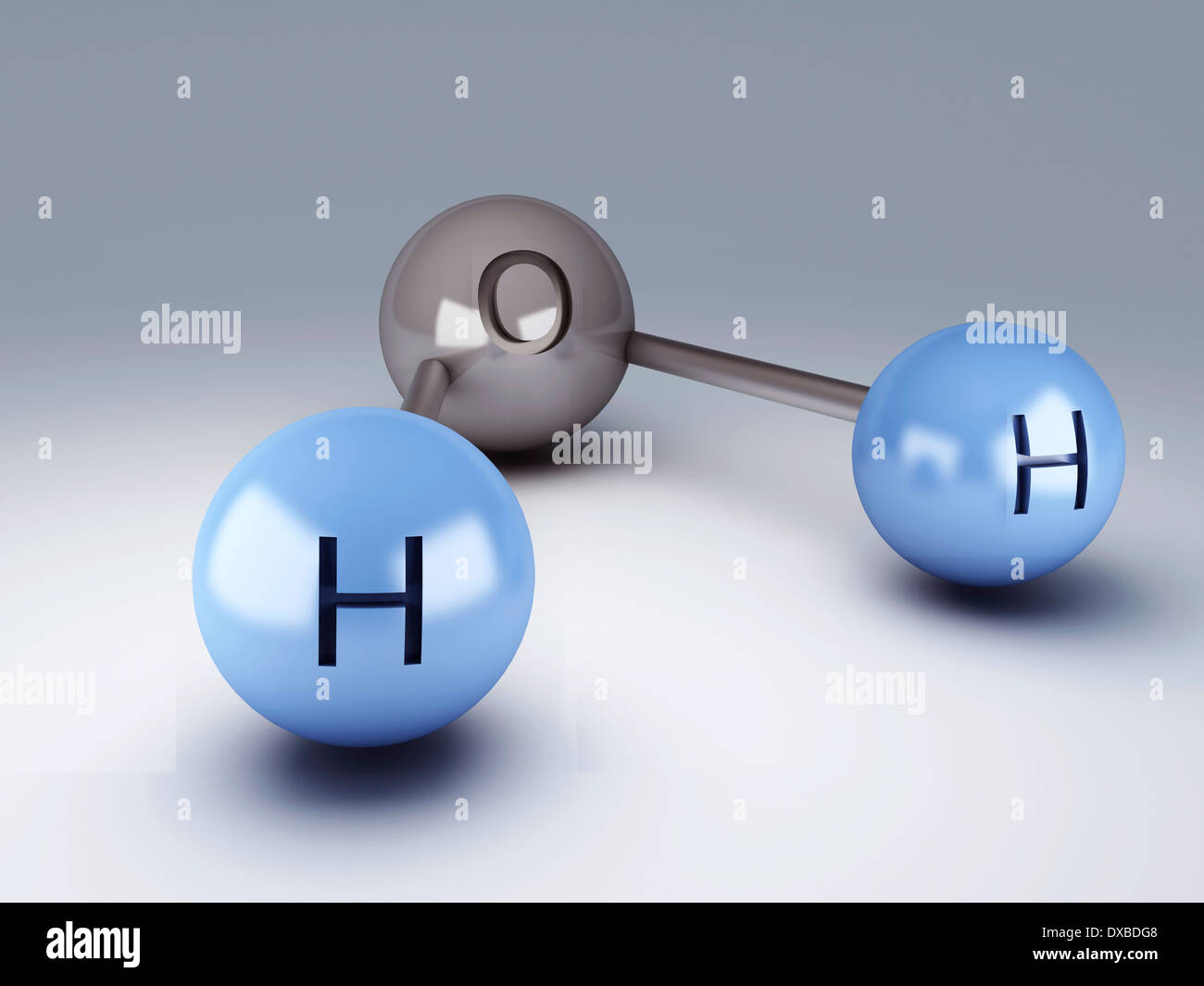
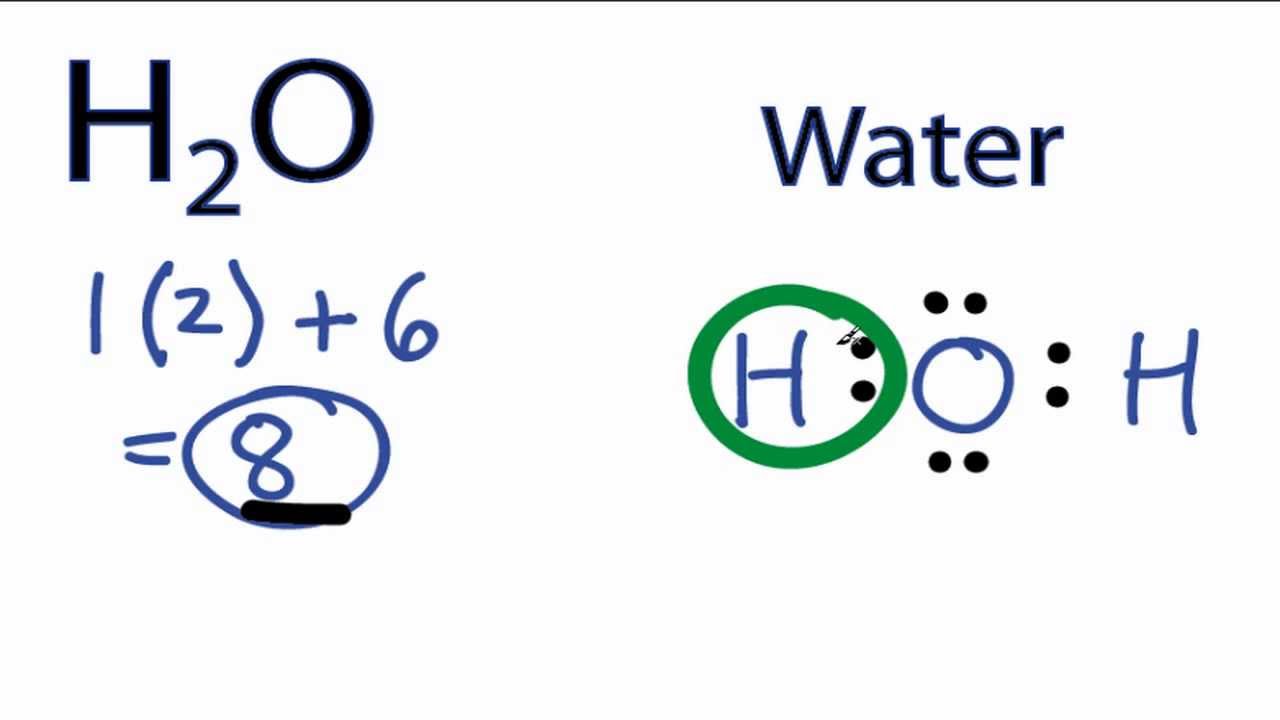H20 Drawing
H20 Drawing - Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other. 22k views 1 year ago. In order to find the total valence electrons in h2o molecule, first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Drawing the lewis dot structure for h2o and answer the questions below. • how to draw lewis. See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. In this video we look at the electron geometry for water (h2o). Web 6 steps to draw the lewis structure of h2o. Web steps of drawing h2o lewis structure.
Because the water molecule has four electron domains (the two hydrogen. For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. These diagrams are a helpful tool in chemistry to understand the bonding behavior of atoms and how they interact with each other. First, determine the total number of valence electrons. Look at the questions before watching the video. On this picture you can see tetrahedral shapes of water, ammonia and methane. Since h 2 o has two hydrogen atoms and one oxygen. Look for the total valence electrons: For h₂o, o must be the central atom.
Make sure you have two hydrogens and one oxygen in h 2 o! For h₂o, o must be the central atom. Web drawing the lewis structure of h2o helps us visualize the arrangement of atoms and valence electrons in the molecule. Web what is the lewis structure of water h2o? You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. Because the water molecule has four electron domains (the two hydrogen. Drawing the lewis structure for water. It is four for one water (h2o) molecule according to the octet rule. Look for how many electrons are needed:
Chemistry model of molecule water H2O scientific elements. Integrated
I also go over hybridization, shape and bond angle. In this video we look at the electron geometry for water (h2o). It is eight to form a single h2o molecule. Because the water molecule has four electron domains (the two hydrogen. Hydrogen peroxide, also called dihydrogen dioxide.
Lewis Dot Diagram For H2o Free Diagram For Student
Look for how many electrons are needed: Water has tetrahedral shape, or to be more precise, bent shape. Hence, hydrogen has one valence electron and oxygen has six valence electrons. Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other. Since h 2 o has two hydrogen atoms and one oxygen.
H2O Chemical Medical Formula for Water Molecula in Blue Color. Simple
Web steps of drawing h2o lewis structure. It is four for one water (h2o) molecule according to the octet rule. You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. Water has tetrahedral shape, or to be more precise, bent shape. Web explore the molecular structure and geometry.
Draw Step By Step The Lewis Structure For Water (H2O)
In order to find the total valence electrons in h2o molecule, first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other. Drawing the lewis dot structure for h2o and answer the questions below. Hence,.
H2O Lewis Structure, Molecular Geometry, and Hybridization
A common error it to put two oxygen atoms and one hydrogen making ho 2. On this picture you can see tetrahedral shapes of water, ammonia and methane. A simple notation used to represent valence electrons in an atom is called lewis. Find the total valence electrons in h2o molecule. 22k views 1 year ago.
H2o water molecule Royalty Free Vector Image VectorStock
Look for the total valence electrons: (valence electrons are the electrons that are present in the outermost orbit of any. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. For h₂o, o must be the central atom. But it is better to say that methane has tetrahedral shape,.
H2O Water molecule model and chemical formula Digital Art by Peter
For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. Let's do the lewis structure for h2o2: Since h 2 o has two hydrogen atoms and one oxygen. It is four for one water (h2o) molecule according to the octet rule. Web explore the molecular structure and geometry of water, one.
H2O Molecule 3d illustration Stock Photo 67864280 Alamy
For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. It allows us to understand the bonding and electron distribution, which are crucial for understanding the chemical behavior of.
H2o water molecule model chemical formula Vector Image
Since h 2 o has two hydrogen atoms and one oxygen. Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other. Web watch the video of dr. It is four for one water (h2o) molecule according to the octet rule. Remember that hydrogen only needs two electrons to have a full outer.
Water Lewis Structure How to Draw the Lewis Structure for Water YouTube
• how to draw lewis. I also go over hybridization, shape and bond angle. Hence, hydrogen has one valence electron and oxygen has six valence electrons. (valence electrons are the electrons that are present in the outermost orbit of any. Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other.
In This Video We Look At The Electron Geometry For Water (H2O).
Hydrogen peroxide, also called dihydrogen dioxide. Drawing the lewis dot structure for h2o and answer the questions below. Look for how many electrons are needed: (valence electrons are the electrons that are present in the outermost orbit of any.
840 Views 4 Years Ago Lewis Structure (Chemistry) How To Draw Lewis Structure For H2O Water Lewis Structure:
Water has tetrahedral shape, or to be more precise, bent shape. For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. These diagrams are a helpful tool in chemistry to understand the bonding behavior of atoms and how they interact with each other. Web lewis structure of water molecule contains two single bonds.
Hence, Hydrogen Has One Valence Electron And Oxygen Has Six Valence Electrons.
Web 6 steps to draw the lewis structure of h2o. Let's do the lewis structure for h2o2: It allows us to understand the bonding and electron distribution, which are crucial for understanding the chemical behavior of water. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure.
Determine The Total Number Of Electrons In The Valence Shells Of Hydrogen And Oxygen Atoms.
In this article, we will delve into the details of the h2o lewis structure, including how to draw it, its properties, and faqs. Web here, we need to understand how the lewis structure is drawn for the h2o molecule: Web because of the two lone pairs, h 2 o will have a bent molecular geometry and it will be a polar molecule. Since h 2 o has two hydrogen atoms and one oxygen.