Hcn 3D Drawing
Hcn 3D Drawing - H + c + n =1 + 4 + 5 = 10. Drawing the lewis structure for hcn. Average rating 3.4 / 5. Web 6 steps to draw the lewis structure of hcn. Web draw the lewis structure of hcn and then determine its electron domain and molecular geometries. Web hcn molecular geometry. In order to draw the lewis structure of hcn, first of all you have to find the total number of valence electrons present in the hcn molecule. How does molecule shape change with different numbers of bonds and electron pairs? Count the valence electrons you can use. Put the least electronegative atom c in the middle with h and cl on either side.
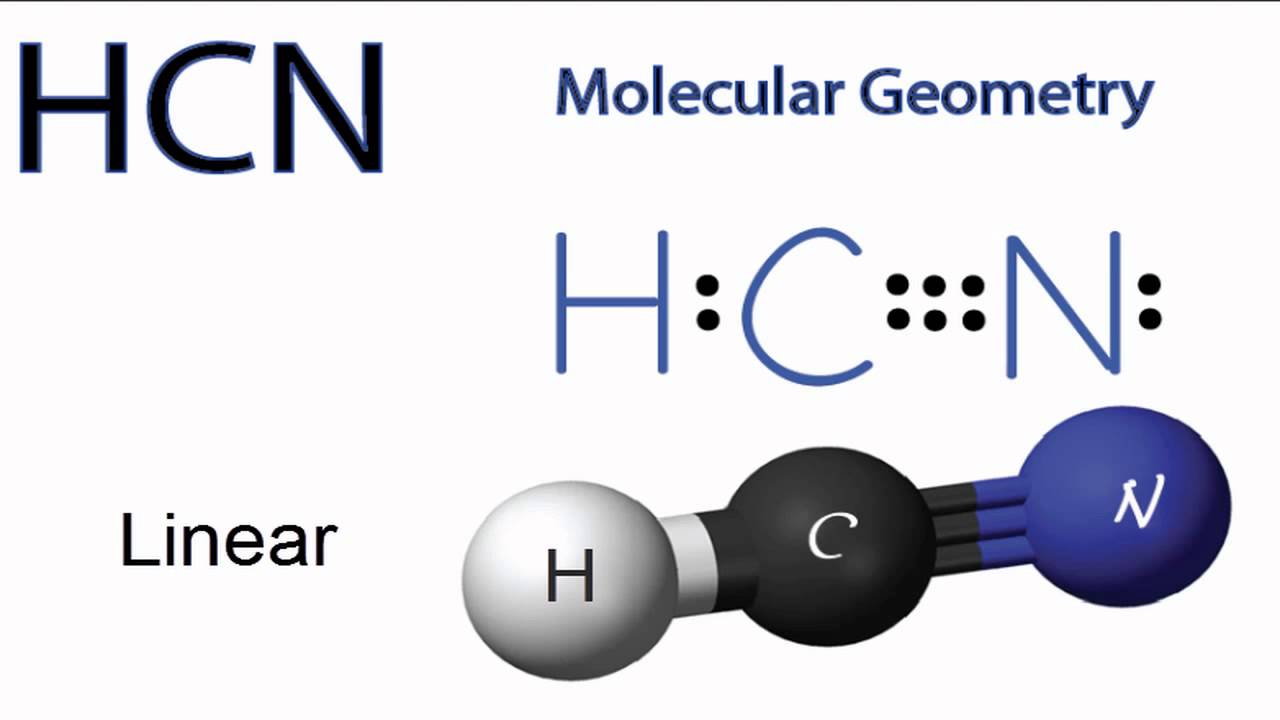
Web hcn molecular geometry. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Hcn is a linear molecule, since it has only two electron groups and there are no lone pairs on the central carbon atom. There is a total of 1 + 4 + 5 = 10 valence electrons, and we use four of them to make the bonds. Use the following guidelines to draw them correctly: The easiest way to find the molecular geometry of any compound is with the help of the vsepr theory. This tutorial will help you deal with the lewis structure. Web hcn geometry and hybridization carbon is the central atom, so we can draw the skeletal structure: For bonds lying in the plane of the paper, use a regular solid line. How does molecule shape change with different numbers of bonds and electron pairs?
Verified solution this video solution was recommended by our tutors as helpful for the problem above Drawing the lewis structure for hcn. 80k views 10 years ago. Web explore molecule shapes by building molecules in 3d! Drawing, structure and detailed explanations. Here's how to do it. The electron geometry for the hydrogen. Web 6 steps to draw the lewis structure of hcn. #3 calculate and mark formal charges on the atoms, if required. Use the following guidelines to draw them correctly:
HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw
Drawing the lewis structure for hcn. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. #2 mark.
HCN Molecular Geometry YouTube
Web hcn molecular geometry. Verified solution this video solution was recommended by our tutors as helpful for the problem above Hcn is a linear molecule, since it has only two electron groups and there are no lone pairs on the central carbon atom. Calculate the total number of valence electrons. Molview consists of two main parts, a structural formula editor.
Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule
Put the least electronegative atom c in the middle with h and cl on either side. Web 12k views 1 year ago. The electron geometry for the hydrogen. Add these electrons to give every atom an octet. Molview consists of two main parts, a structural formula editor and a 3d model viewer.
Grimy Trend Understanding Hcn Lewis Structure Bonds
#5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure. Web use these steps to correctly draw the hcn lewis structure: Hydrogen cyanide has geometry like ax2 molecule, where a is the central atom and x is the number of atoms bonded with the central atom. The electron geometry for the hydrogen. There.
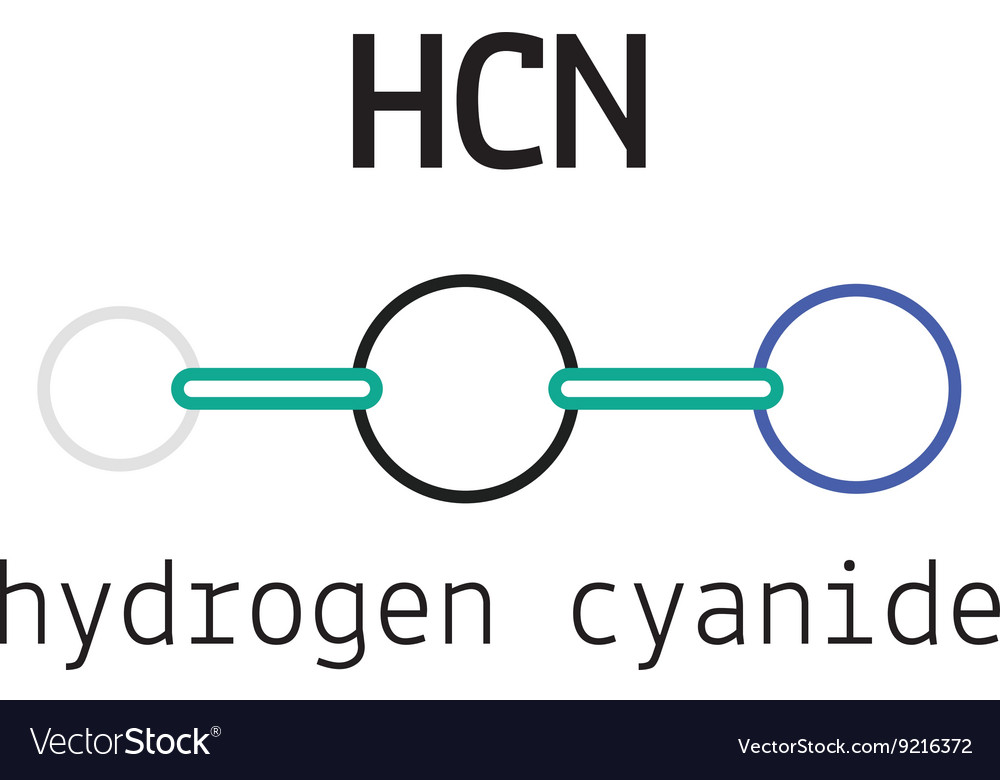
Hcn hydrogen cyanide molecule Royalty Free Vector Image
Web click on a star to rate it! Add these electrons to give every atom an octet. 14k views 10 years ago. Web lewis structure is the basis of any element and here we will discuss how to draw the lewis structure of hcn, along with key information on hcn’s total valence electrons, formal charge, 3d model, bond angle, and.
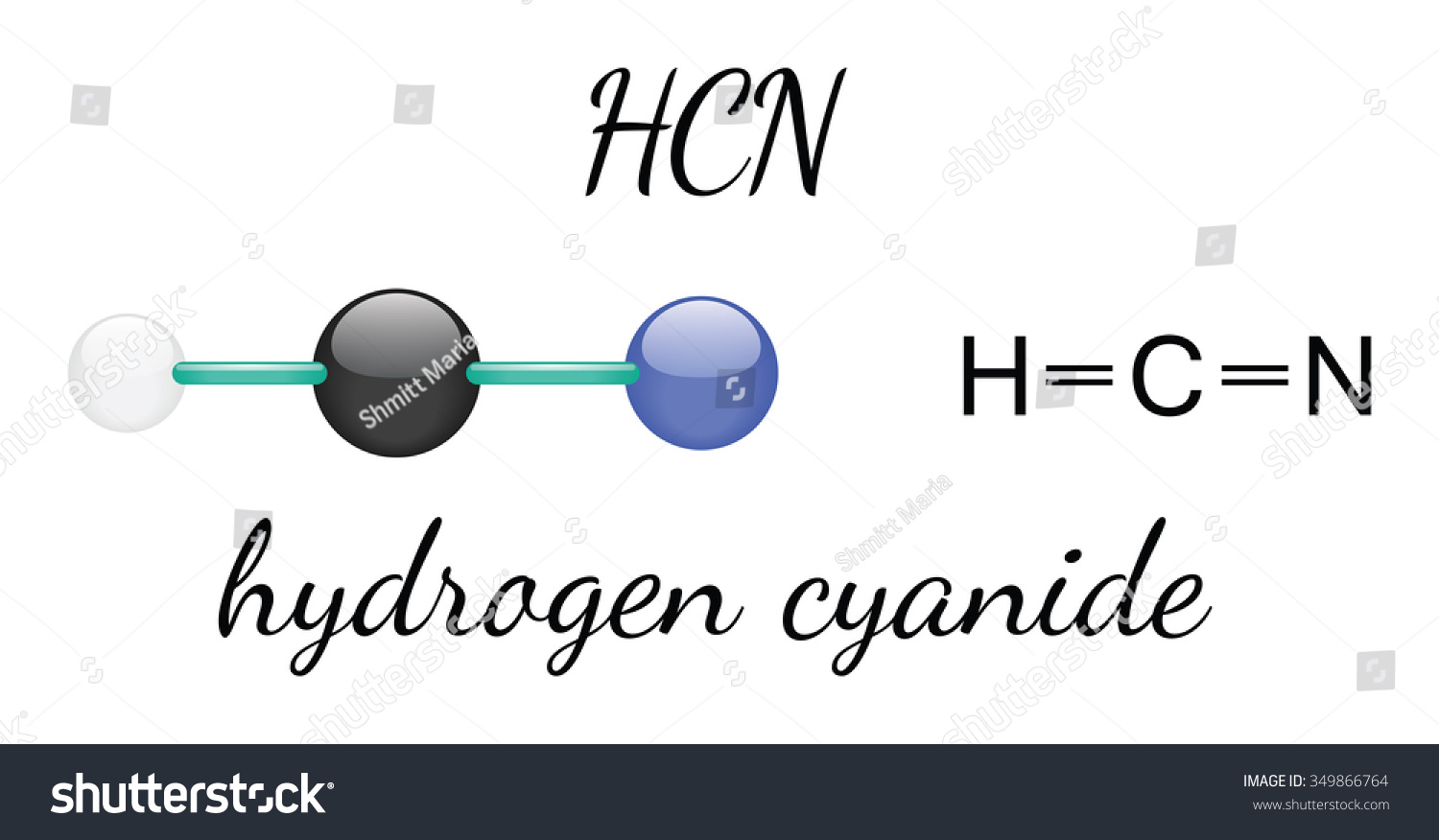
Hcn Hydrogen Cyanide 3d Molecule Isolated Stock Vector 349866764
#5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure. H + c + n =1 + 4 + 5 = 10. Find out by adding single, double or triple bonds and lone pairs to the central atom. #3 calculate and mark formal charges on the atoms, if required. Web explore molecule shapes.
HCN 3D Model Video YouTube
How does molecule shape change with different numbers of bonds and electron pairs? H + c + n =1 + 4 + 5 = 10. Put the least electronegative atom c in the middle with h and cl on either side. For bonds lying in the plane of the paper, use a regular solid line. April 3, 2022 by aditi.
HCN (Hydrogen cyanide) Molecular Geometry & Bond Angles YouTube
For bonds lying in the plane of the paper, use a regular solid line. There is a total of 1 + 4 + 5 = 10 valence electrons, and we use four of them to make the bonds. Web there are 4 steps to solve this one. Add these electrons to give every atom an octet. #5 repeat step 4.
Hcn hydrogen cyanide molecule Royalty Free Vector Image
Hydrogen cyanide has geometry like ax2 molecule, where a is the central atom and x is the number of atoms bonded with the central atom. #3 calculate and mark formal charges on the atoms, if required. Web draw the lewis structure of hcn and then determine its electron domain and molecular geometries. Web hcn geometry and hybridization carbon is the.
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
Web hcn molecular geometry. Drawing the lewis structure for hcn. Hydrogen cyanide has geometry like ax2 molecule, where a is the central atom and x is the number of atoms bonded with the central atom. In order to draw the lewis structure of hcn, first of all you have to find the total number of valence electrons present in the.
In This Article, “Hcn Hybridization” , Hybridization, Lewis Structure, Bond Connectivity Of Hydrogen Cyanide With Detailed Explanation Are Discussed Briefly.
For bonds lying in the plane of the paper, use a regular solid line. Web explore molecule shapes by building molecules in 3d! Web lewis structure is the basis of any element and here we will discuss how to draw the lewis structure of hcn, along with key information on hcn’s total valence electrons, formal charge, 3d model, bond angle, and 3d sketch. #2 mark lone pairs on the atoms.
Here, The Given Molecule Is Hcn.
Drawing the lewis structure for hcn. Drawing, structure and detailed explanations. This tutorial will help you deal with the lewis structure. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then.
Find Out By Adding Single, Double Or Triple Bonds And Lone Pairs To The Central Atom.
Use the following guidelines to draw them correctly: April 3, 2022 by aditi roy. Count the valence electrons you can use. Hydrogen cyanide has geometry like ax2 molecule, where a is the central atom and x is the number of atoms bonded with the central atom.
Web Draw The Lewis Structure Of Hcn And Then Determine Its Electron Domain And Molecular Geometries.
In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. And to find that we need to find the molecular geometry of the compound. Web 6 steps to draw the lewis structure of hcn. How does molecule shape change with different numbers of bonds and electron pairs?