How Many Bonds Will Carbon Form
How Many Bonds Will Carbon Form - Web if carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even more stable. In a single bond, two carbon atoms share one pair of. In the 1970s, scientists made an. This means that it has six protons and six electrons. A bond composed of two electrons, one from each of. Web among chemistry’s most fundamental concepts is that carbon is tetravalent and forms four bonds to other atoms. Web atoms of different elements. Web carbon has four such sharable electrons of its own, so it tends to form four bonds to other atoms. Web carbon can form four covalent bonds to create an organic molecule. And when it comes to aromaticity, benzene’s.
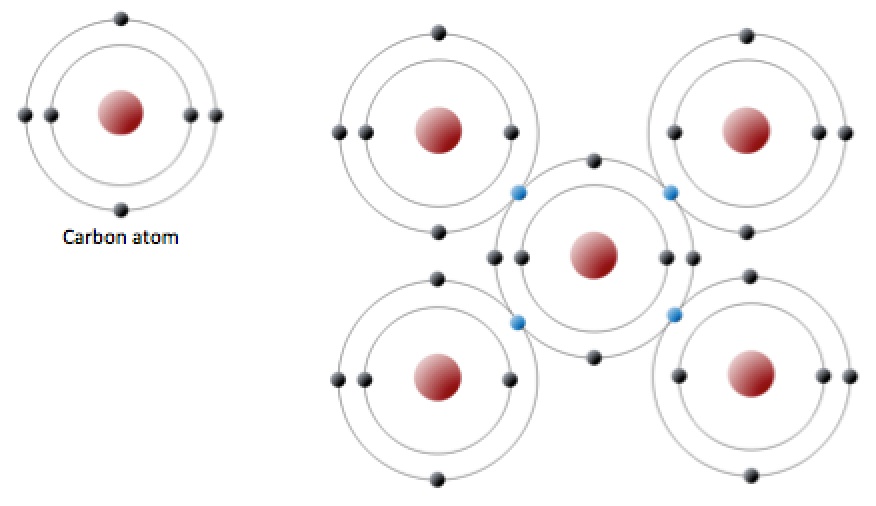
Web carbon is unique and found in all living things because it can form up to four covalent bonds between atoms or molecules. Web use this gcse chemistry study guide to revise the structure of the carbon atom and more. Web the carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural. A bond composed of two electrons, one from each of. Web how many bonds? This enables carbon to share four. Web as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. Web atoms of different elements. Web carbon has four such sharable electrons of its own, so it tends to form four bonds to other atoms. The atomic number of carbon is 6.
•br br submit your choice when you are. Web as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. 0 how many bonds will a carbon atom form with four bromine atoms? Web the carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural. Web because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. A bond composed of two electrons, one from each of. Web carbon can form four covalent bonds to create an organic molecule. Form long c −c chains, with differing substitution along that chain. But that rule doesn’t always hold. And when it comes to aromaticity, benzene’s.
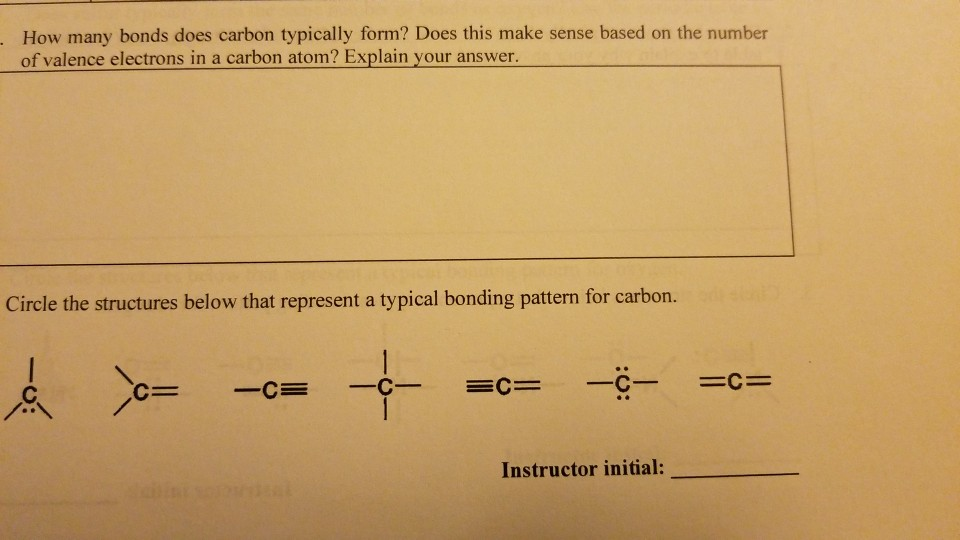
Solved How many bonds does carbon typically form? Does this
Web use this gcse chemistry study guide to revise the structure of the carbon atom and more. These can be nonpolar or polar covalent bonds,. Web among chemistry’s most fundamental concepts is that carbon is tetravalent and forms four bonds to other atoms. Giant covalent substances contain many atoms joined together by covalent bonds. This means that it has six.
PPT Organic Chemistry Functional Groups PowerPoint Presentation
Carbon atoms can form four covalent bonds, consisting of any one of the following: Web modeling lonic and covalent bonds part 1: Web the carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural. A very common ring structure contains.
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
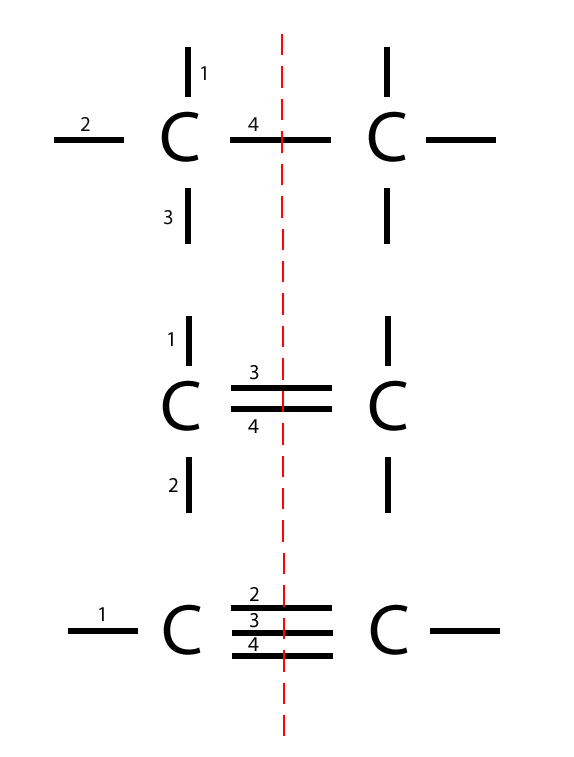
Web modeling lonic and covalent bonds part 1: [1] the most common form is the single bond: Web atoms of different elements. Web carbon has four such sharable electrons of its own, so it tends to form four bonds to other atoms. Carbon can form single, double, or even triple bonds with other carbon atoms.
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
Carbon can form single, double, or even triple bonds with other carbon atoms. Web modeling lonic and covalent bonds part 1: [1] the most common form is the single bond: There is only a small energy. Web atoms of different elements.
2.2 Bonding and Lattices Physical Geology
Web carbon has four such sharable electrons of its own, so it tends to form four bonds to other atoms. Web modeling lonic and covalent bonds part 1: A very common ring structure contains six carbon. These can be nonpolar or polar covalent bonds,. [1] the most common form is the single bond:
PPT Biochemistry PowerPoint Presentation, free download ID89333
The atomic number of carbon is 6. This means that it has six protons and six electrons. Web carbon can also catenate; Web as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. Web the carbon atom has unique properties that allow it to form covalent bonds to as many as.
How Many Single Bonds Can Carbon Form fredhughesdesign
Web how many bonds? In a single bond, two carbon atoms share one pair of. Web carbon can also catenate; The simplest carbon molecule is methane (ch 4 ), depicted here. Web modeling lonic and covalent bonds part 1:
how many bonds can carbon form YouTube
Carbon can form single, double, or even triple bonds with other carbon atoms. [1] the most common form is the single bond: This enables carbon to share four. Carbon atoms can form four covalent bonds, consisting of any one of the following: Web the carbon atom has unique properties that allow it to form covalent bonds to as many as.
Solved Carbon forms bonds by sharing an electron in one of
This means that it has six protons and six electrons. Carbon atoms can form four covalent bonds, consisting of any one of the following: There is only a small energy. A very common ring structure contains six carbon. Web carbon can form four covalent bonds to create an organic molecule.
How Many Single Bonds Can Carbon Form fredhughesdesign
A bond composed of two electrons, one from each of. Web carbon is unique and found in all living things because it can form up to four covalent bonds between atoms or molecules. Web the carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to.
And When It Comes To Aromaticity, Benzene’s.
Carbon atoms can form four covalent bonds, consisting of any one of the following: There is only a small energy. These can be nonpolar or polar covalent bonds,. Web if carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even more stable.
0 How Many Bonds Will A Carbon Atom Form With Four Bromine Atoms?
The simplest carbon molecule is methane (ch 4 ), depicted here. This means that it has six protons and six electrons. But that rule doesn’t always hold. [1] the most common form is the single bond:
Web These Four Electrons Can Be Gained By Forming Four Covalent Bonds, As Illustrated Here For Carbon In Ccl 4 (Carbon Tetrachloride) And Silicon In Sih 4 (Silane).
Web as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. Web because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. •br br submit your choice when you are. In a single bond, two carbon atoms share one pair of.
Web Carbon Is Unique And Found In All Living Things Because It Can Form Up To Four Covalent Bonds Between Atoms Or Molecules.
Web use this gcse chemistry study guide to revise the structure of the carbon atom and more. In the 1970s, scientists made an. A very common ring structure contains six carbon. This enables carbon to share four.