How Many Hydrogen Bonds Can A Water Molecule Form
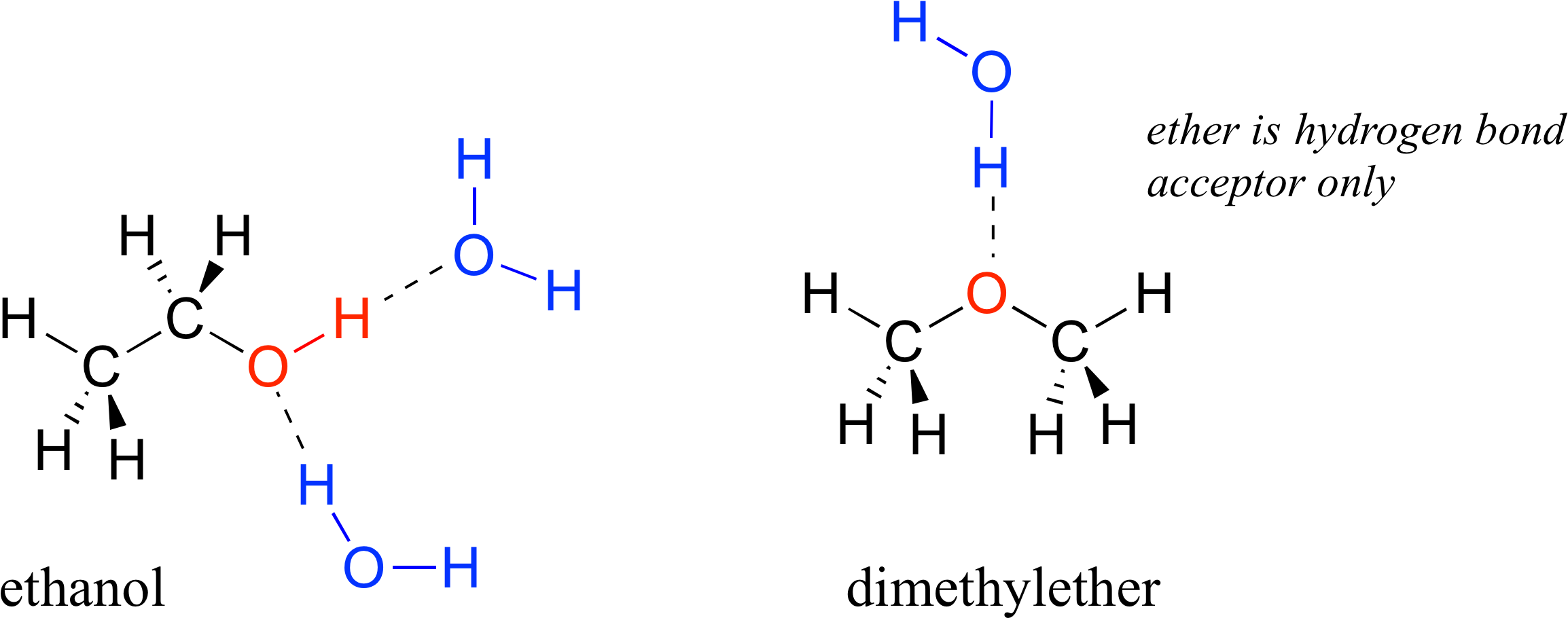
How Many Hydrogen Bonds Can A Water Molecule Form - Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: That means that every hydrogen. Web answer (1 of 2): Hydrogen bond is an intermolecular force (force between molecules). Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. One molecule of water can make four. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web the dynamic interactions of water molecules. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Oxygen is highly electronegative, which creates a partial negative charge on one end of.
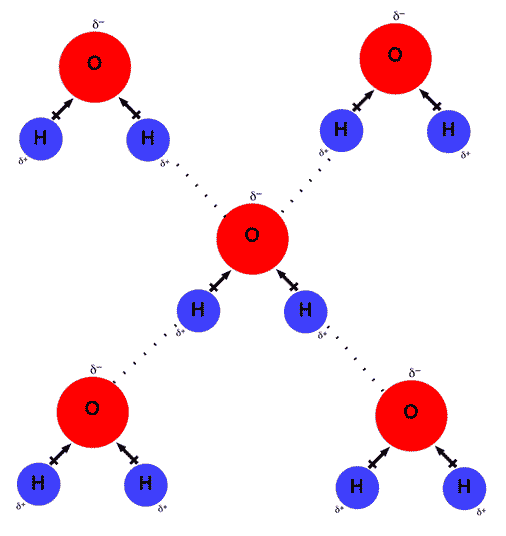
Web the dynamic interactions of water molecules. Web water is can ideally model of hydrogen stick. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. One molecule of water can make four. Web lewis structure of h 2 o indicating bond angle and bond length. Hydrogen bond is an intermolecular force (force between molecules). Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Each water molecule is surrounded by four neighboring h 2 os.
Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. One molecule of water can make four. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Hydrogen bond is an intermolecular force (force between molecules). Web answer (1 of 2): Web the molecular formula for water is h 2 o.
Download How Many Hydrogen Bonds Can A Water Molecule Form Hydrogen
Therefore one single water molecule does not contain any. One molecule of water can make four. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: Web water is can ideally model of hydrogen stick. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that.
Water
Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Therefore one single water molecule does not contain any. Web how many hydrogen bonds can a single water molecule form? Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes.
😎 What properties of water make it essential to life. Why Water Is
Web water is can ideally model of hydrogen stick. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web how.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
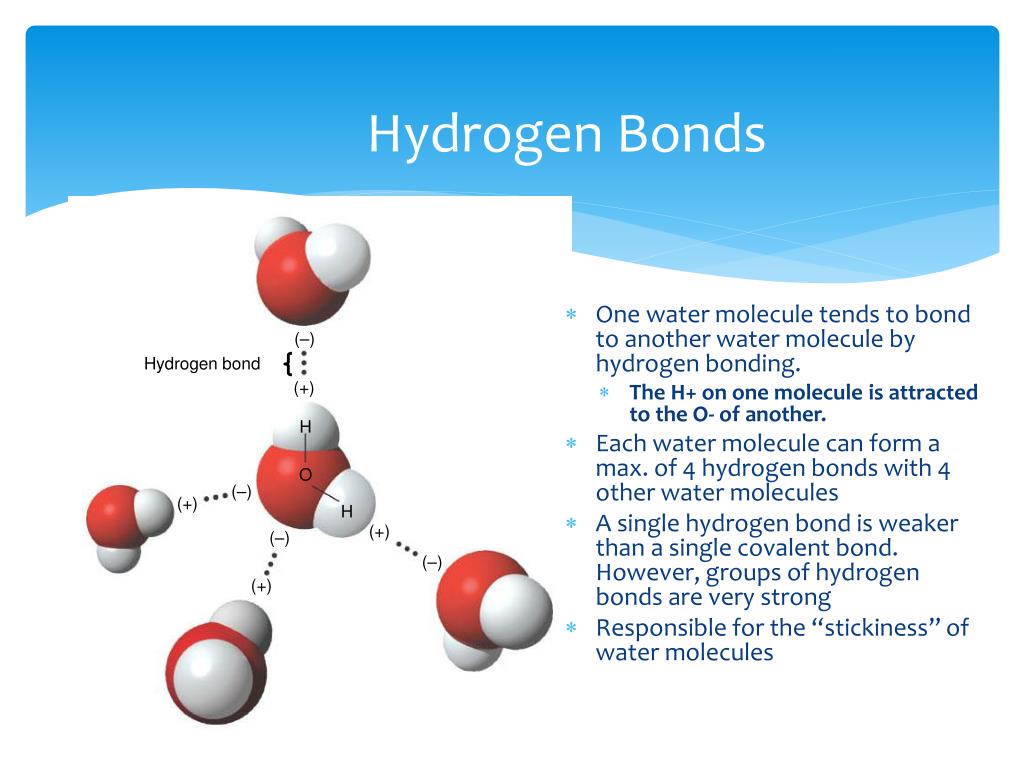
Each water molecule is surrounded by four neighboring h 2 os. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web the dynamic interactions of water molecules. Web a hydrogen bond will form between the negative oxygen of one molecule with the positive hydrogen of another molecule. Both an oxygen atom and.
Pin on Hydrogen
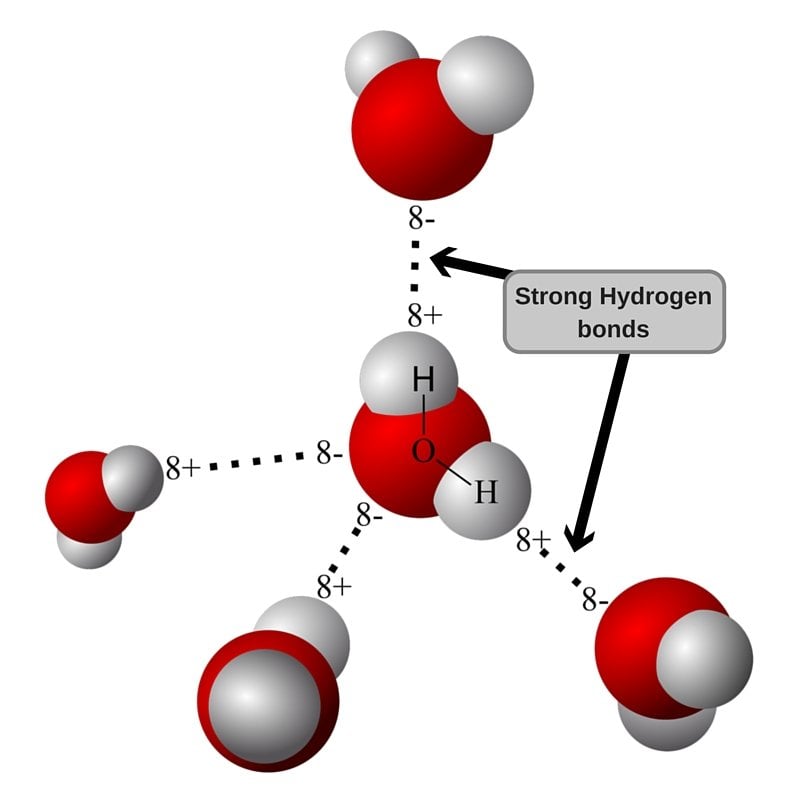
Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Web the dynamic interactions of water molecules. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Oxygen is highly electronegative, which creates a partial negative charge on one end of. Web water is capable of participating in 4 hydrogen bonds.
PPT Properties of Water PowerPoint Presentation, free download ID
Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Oxygen is highly electronegative, which creates a partial negative charge on one end of. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Web up to.
How many hydrogen bonds are attached to each water molecule in a solid
Web answer (1 of 2): Web lewis structure of h 2 o indicating bond angle and bond length. Both an oxygen atom and 2 hydrogen atoms in one molecule. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web how many hydrogen bonds can a single water molecule form?
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
One molecule of water can make four. Each water molecule is surrounded by four neighboring h 2 os. Hydrogen bond is an intermolecular force (force between molecules). That means that every hydrogen. Web water is can ideally model of hydrogen stick.
Water Polar Covalent Bond My XXX Hot Girl
Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. Hydrogen bond is an intermolecular force (force between molecules). Therefore one single water molecule does not contain any. Water (h 2 o) is a simple triatomic bent molecule with c 2v.
Why Do Fingers/Hands Stick To Ice? » Science ABC
Oxygen is highly electronegative, which creates a partial negative charge on one end of. Web the dynamic interactions of water molecules. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be.
Therefore One Single Water Molecule Does Not Contain Any.
Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web a hydrogen bond will form between the negative oxygen of one molecule with the positive hydrogen of another molecule. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them.
Web Water Is Capable Of Participating In 4 Hydrogen Bonds At Once, Granted It Only Does This When It Forms A Perfect Crystal Structure.
Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Hydrogen bond is an intermolecular force (force between molecules). Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules:
In H 2 O, Only Two Of The Six.
Web the molecular formula for water is h 2 o. Both an oxygen atom and 2 hydrogen atoms in one molecule. Web the dynamic interactions of water molecules. One molecule of water can make four.
Each Water Molecule Is Surrounded By Four Neighboring H 2 Os.
Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web water is can ideally model of hydrogen stick. Oxygen is highly electronegative, which creates a partial negative charge on one end of. Web answer (1 of 2):