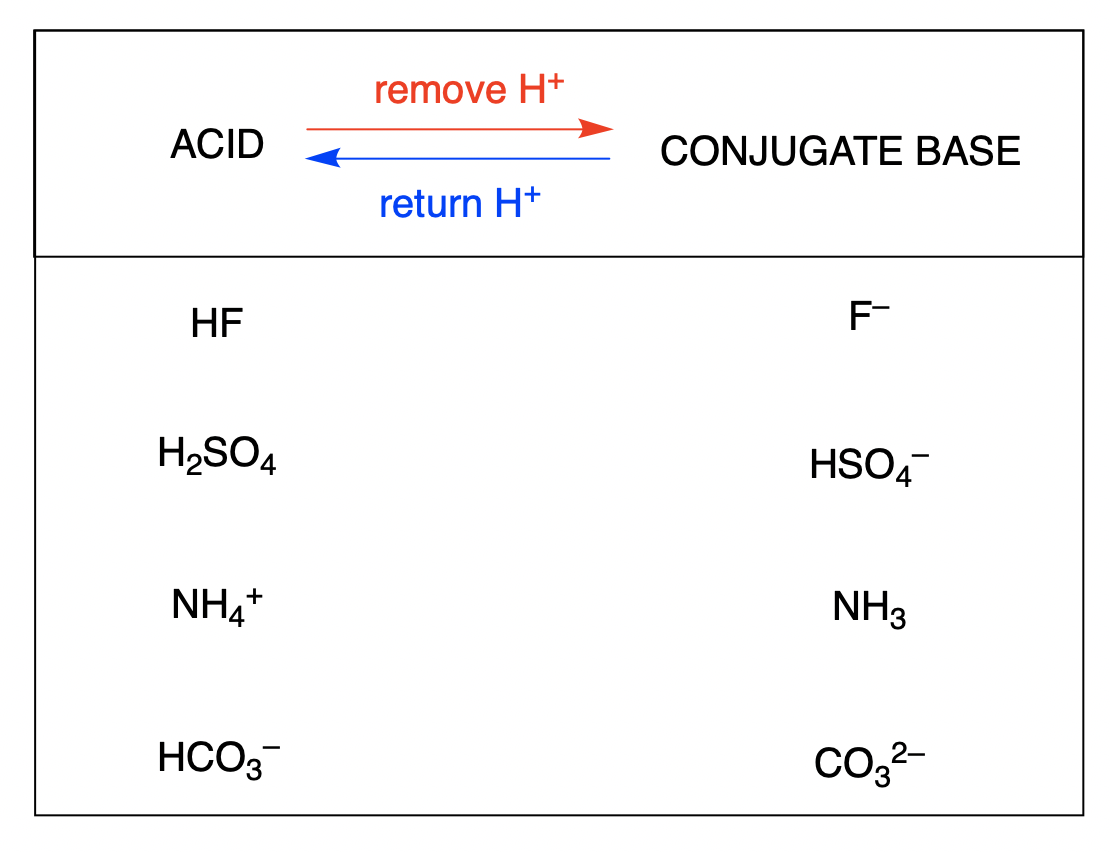
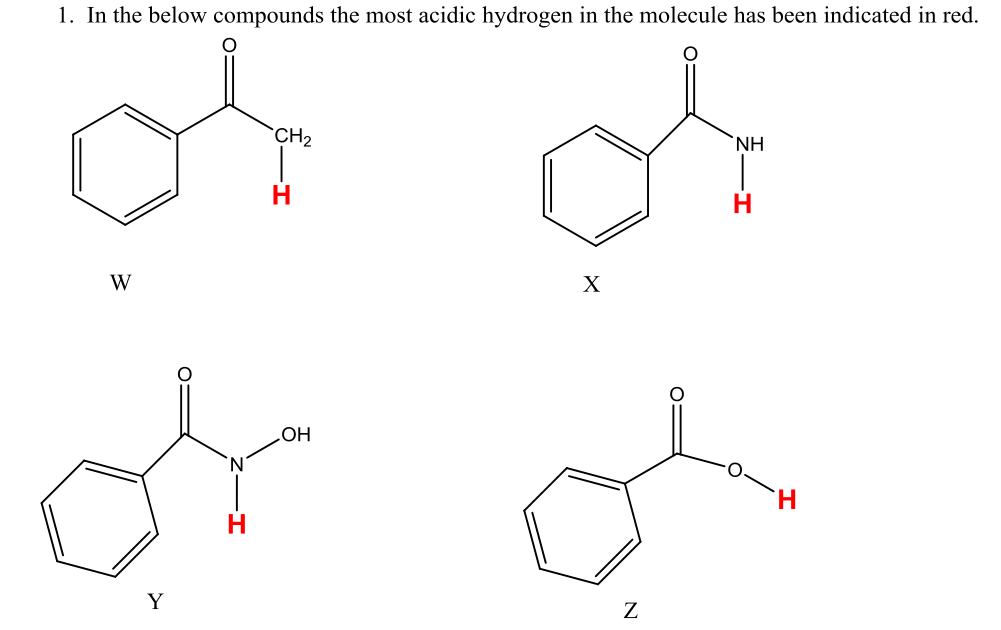
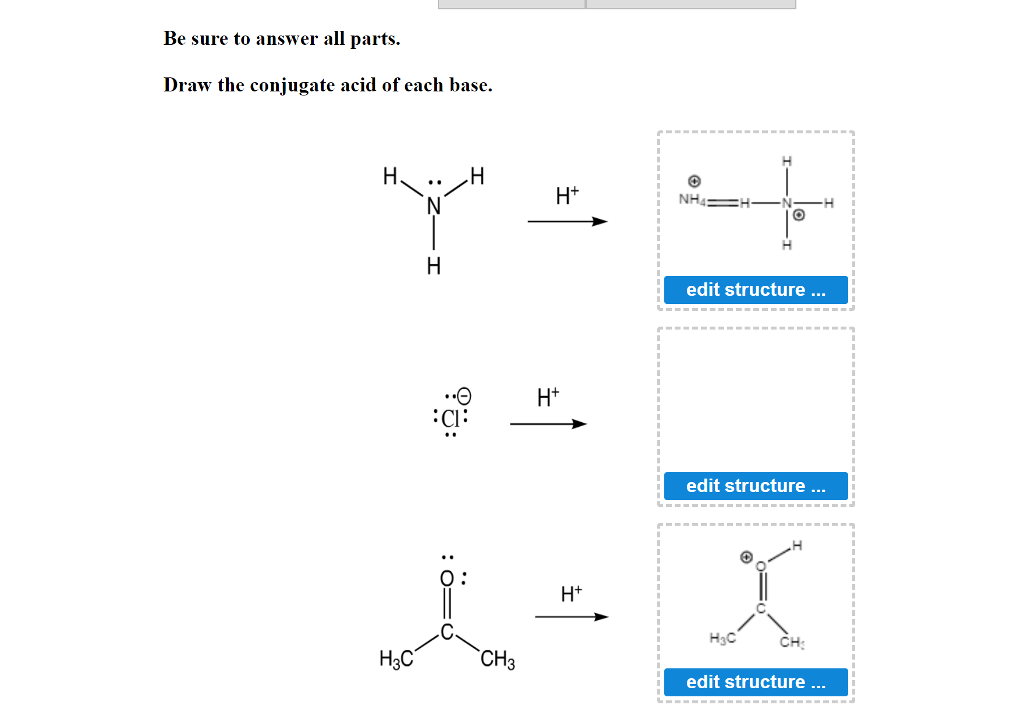
How To Draw Conjugate Base
How To Draw Conjugate Base - Web stabilization of a conjugate base: Web introduction to conjugate acids and bases. Web so if you add a proton to oh minus, you get h2o. So water is the conjugate, oops. Hcl + h 2 o ↔ cl − + h 3 o +. Draw the conjugate bases for each acid. If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have. An important requirement of this mechanism is the. Web draw the conjugate bases and several resonance structures of each. Pka trends for allylic and propargylic (a to an alkyne) systems:
Did you know that everything is made out of chemicals? Web let's draw the conjugate base for acetic acid. Hcl + h 2 o ↔ cl − + h 3 o +. I'm writing conjugate base here, but it's really the conjugate acid, right. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. How a conjugate base is stabilized by the inductive effect. A conjugate acid is formed when a proton is added to a base, and a conjugate base is. Web 📗 need help with chemistry? Let us take the example of bicarbonate ions reacting with water to create. \(\mathrm{{\color{blue} nh_3} + h_2o \rightleftharpoons {\color{red} nh_4^+}.
Web draw the structure of ascorbate, the conjugate base of ascorbic acid, then draw a second resonance contributor showing how the negative charge is delocalized to. So water is the conjugate, oops. Web let's draw the conjugate base for acetic acid. Web draw the conjugate bases and several resonance structures of each. Web in this mechanism, the first step is formation of the conjugate base of the metal complex by deprotonation of one of its ligands. Web so if you add a proton to oh minus, you get h2o. How a conjugate base is stabilized by the inductive effect. Draw the conjugate bases for each acid. The stronger an acid, the weaker. I'm writing conjugate base here, but it's really the conjugate acid, right.
How to typset/draw conjugate acids and bases
Here, the chloride anion, cl. Let us take the example of bicarbonate ions reacting with water to create. Web let's draw the conjugate base for acetic acid. Web introduction to conjugate acids and bases. How a conjugate base is stabilized by the inductive effect.
Conjugate acidbase pairs YouTube
An important requirement of this mechanism is the. Web 📗 need help with chemistry? Hcl + h 2 o ↔ cl − + h 3 o +. Web introduction to conjugate acids and bases. The stronger an acid, the weaker.
Chapter 10 Exercises 3 and 4 Drawing Conjugate Acids and Conjugate
Pka trends for allylic and propargylic (a to an alkyne) systems: The stronger an acid, the weaker. A conjugate acid is formed when a proton is added to a base, and a conjugate base is. Web in this mechanism, the first step is formation of the conjugate base of the metal complex by deprotonation of one of its ligands. Web.
CHEM112 5 6 drawing conjugate acids and bases YouTube
Web hx + h 2 o ↔ x − + h 3 o +. Pka trends for allylic and propargylic (a to an alkyne) systems: Draw the conjugate bases for each acid. \(\mathrm{{\color{blue} nh_3} + h_2o \rightleftharpoons {\color{red} nh_4^+}. Created by sal khan.chemistry on khan academy:
5.1 AcidBase Definitions & Conjugate AcidBase Pairs General
Here, the chloride anion, cl. The stronger an acid, the weaker. Web let's draw the conjugate base for acetic acid. Web stabilization of a conjugate base: For hydrochloric acid (hcl), this reaction becomes:
[Solved] Draw the conjugate base for the acid CH2=CH2. Draw all
Web introduction to conjugate acids and bases. The stronger an acid, the weaker. Pka trends for allylic and propargylic (a to an alkyne) systems: Web draw the conjugate bases and several resonance structures of each. Web so if you add a proton to oh minus, you get h2o.
Acids, bases and salts Learning Lab
The stronger an acid, the weaker. Web draw the structure of ascorbate, the conjugate base of ascorbic acid, then draw a second resonance contributor showing how the negative charge is delocalized to. An important requirement of this mechanism is the. Draw the conjugate bases for each acid. Web so if you add a proton to oh minus, you get h2o.
Enter the Conjugate Base for Each Acid.
Pka trends for allylic and propargylic (a to an alkyne) systems: Created by sal khan.chemistry on khan academy: Draw the conjugate bases for each acid. Web so if you add a proton to oh minus, you get h2o. Web let's draw the conjugate base for acetic acid.
Solved A. Draw the conjugate base of each acid, including
Web let's draw the conjugate base for acetic acid. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. Web so if you add a proton to oh minus, you get h2o. The stronger an acid, the weaker. Web introduction to conjugate acids and bases.
Web So If You Add A Proton To Oh Minus, You Get H2O.
Web in this mechanism, the first step is formation of the conjugate base of the metal complex by deprotonation of one of its ligands. How a conjugate base is stabilized by the inductive effect. Web stabilization of a conjugate base: 885k views 7 years ago.
Here, The Chloride Anion, Cl.
Web hx + h 2 o ↔ x − + h 3 o +. An important requirement of this mechanism is the. Web 📗 need help with chemistry? \(\mathrm{{\color{blue} nh_3} + h_2o \rightleftharpoons {\color{red} nh_4^+}.
Web Draw The Structure Of Ascorbate, The Conjugate Base Of Ascorbic Acid, Then Draw A Second Resonance Contributor Showing How The Negative Charge Is Delocalized To.
Web draw the conjugate bases and several resonance structures of each. A conjugate acid is formed when a proton is added to a base, and a conjugate base is. Web stabilization of a conjugate base: Web let's draw the conjugate base for acetic acid.
Hcl + H 2 O ↔ Cl − + H 3 O +.
Let us take the example of bicarbonate ions reacting with water to create. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. I'm writing conjugate base here, but it's really the conjugate acid, right. The stronger an acid, the weaker.