How To Draw Ionic Lewis Structures
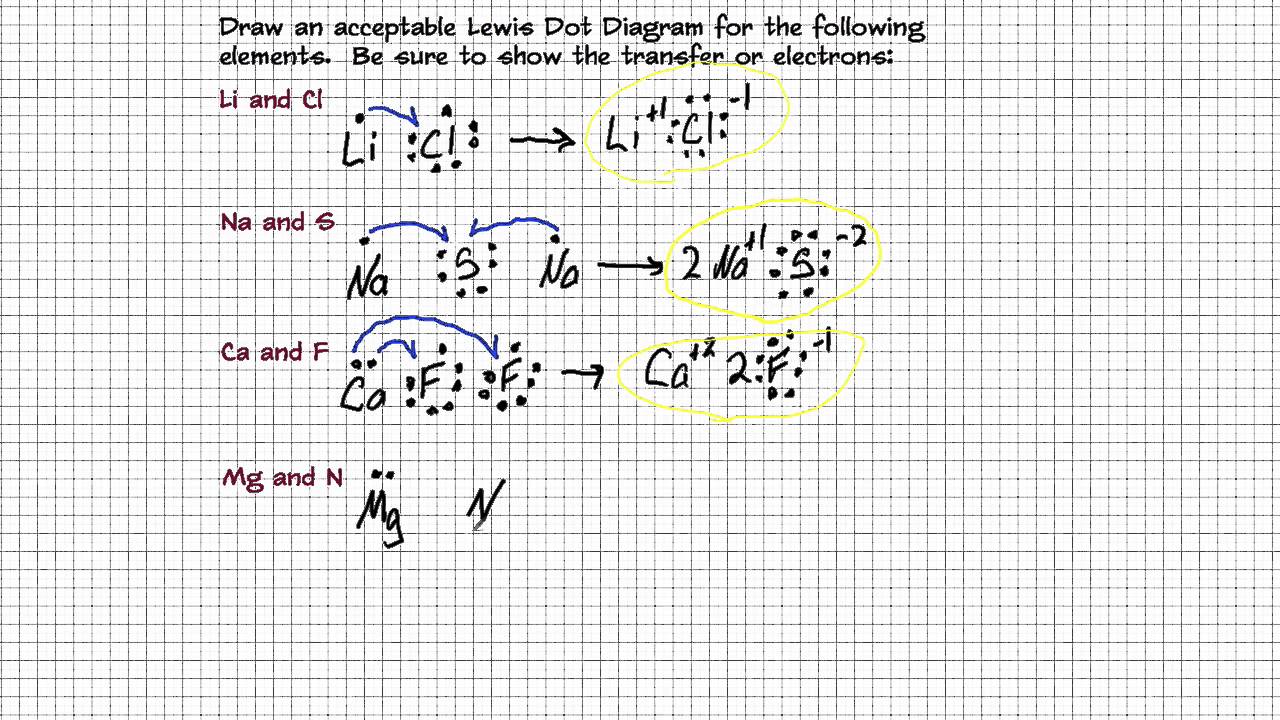
How To Draw Ionic Lewis Structures - What are lewis dot structures used for? Examples include nacl, mgf2, k2o, and al2o3.how to draw lewis structures:. In this case, we can condense the last few steps, since not all of them apply. The tendency to form species that have eight electrons in the valence shell. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. The steps that must be followed while drawing a lewis structure are listed below. Calculate the number of valence electrons: Web ionic compounds can be represented by the lewis dot structures, considering that a positive charge indicates that electrons have been lost. How do you draw the lewis structure for ions? As noted above, formal charge is used as.
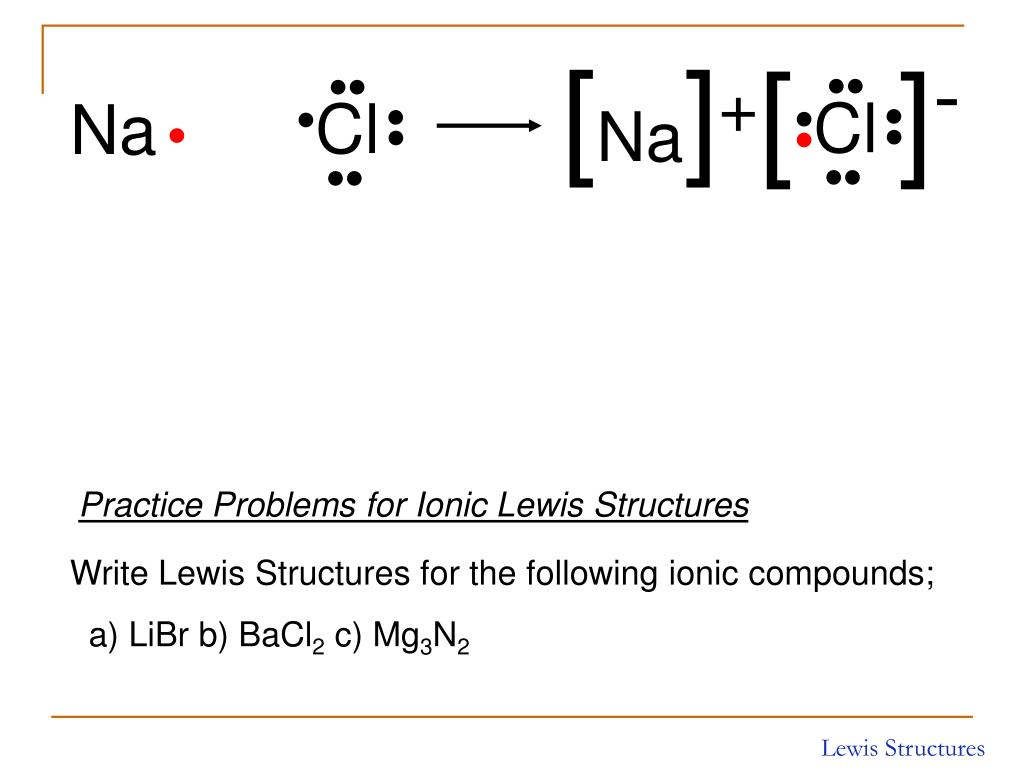
When you draw an ion, don't forget [ ] and a charge. What are lewis dot structures used for? In contrast, a negative charge indicates that additional electrons have been gained. Web shows how to draw lewis dot structures for ionic compounds. Examples include nacl, mgf2, k2o, and al2o3.how to draw lewis structures:. Web draw lewis structures for ionic compounds. What is the lewis structure for #so_2#? Placing a bonding pair of electrons between each pair of bonded atoms gives the following: In this case, we can condense the last few steps, since not all of them apply. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding.
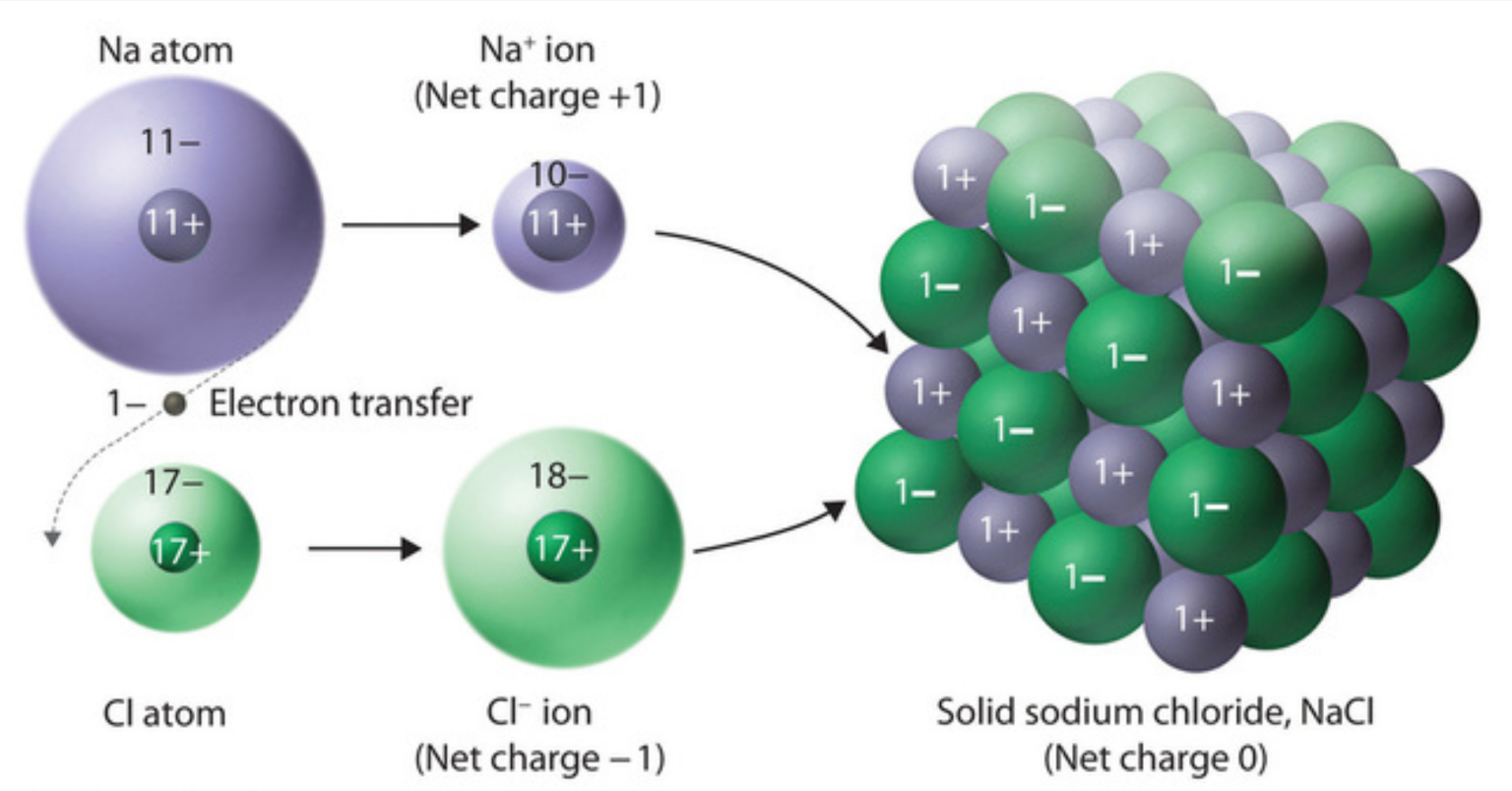
Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Draw the lewis dot structure for the hydrogen atom. How do you draw the lewis structure for ions? When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons. We can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. Web when drawing lewis structures for compounds formed from the combination of a metal/nonmetal, use the list of rules shown below: Ionic bonds are caused by electrons transferring from one atom to another.
Drawing Lewis Dot Diagrams for Ionic Compounds YouTube
Web in this video you’ll learn how to draw lewis dot structures for covalent compounds. Web draw lewis structures for ionic compounds. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Six electrons are used, and 6 are left over. Web in an ionic bond, it is more like one atom donating.
Chemical Bonding Lewis Structures of Ionic Compounds YouTube
Lewis dot structures for ionic compounds are always encased in brackets, with the charge. Web here are the steps to draw a lewis structure. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: We will also go over what the octet rule is and all the e. The video covers the basic lewis structures.
Introduction to Ionic Bonding, Properties of Ionic Compounds, How to
Web in an ionic bond, it is more like one atom donating an electron to the other atom. Web the strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. Web shows how to draw lewis dot structures for ionic compounds. Web in this video you’ll learn how to draw lewis dot structures.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Web when drawing lewis structures for compounds formed from the combination of a metal/nonmetal, use the list of rules shown below: First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of. Web shows how to draw lewis dot structures for ionic compounds. Connect the atoms to each other with single bonds.
How to Draw a Lewis Structure
See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. Since it is often possible to draw more than one valid lewis structure for a molecule or molecular ion, we will need to assess which ones are more plausible or make better chemical sense. We will also go over what the octet.
Molecular Modeling Digital and Analog Middlebury College Chem 103 lab
The video covers the basic lewis structures you'll see in an introductor. How to draw lewis structures. Web ionic lewis dot structures. We will also go over what the octet rule is and all the e. Since hydrogen is in group i it has one (1) valence electron in its shell.
Lewis Dot Structures of Atoms and Ions YouTube
Draw the lewis dot structure for the hydrogen atom. The two ions attract each other according to coulombic interactions. How do you draw the lewis structure for ionic compounds? When discussing the octet rule, we do not consider d or f. Web shows how to draw lewis dot structures for ionic compounds.
Ionic Bonding Explained Discover Tutoring
Web when drawing lewis structures for compounds formed from the combination of a metal/nonmetal, use the list of rules shown below: In electron transfer, the number of electrons lost must equal the number of electrons gained. Cation and anion charges must be on lewis structure to receive full credit (this is an ionic compound). Web a lewis electron dot structure.
How to Draw a Lewis Structure
When atoms have fewer than eight electrons, they tend to react and form more stable compounds. Web in this video you’ll learn how to draw lewis dot structures for covalent compounds. In contrast, a negative charge indicates that additional electrons have been gained. We can draw the lewis structure of any covalent molecule by following the six steps discussed earlier..
How To Draw Lewis Structures For Ionic Compounds slideshare
Cation and anion charges must be on lewis structure to receive full credit (this is an ionic compound). When discussing the octet rule, we do not consider d or f. Lewis dot structures for ionic compounds are always encased in brackets, with the charge. We saw this in the formation of nacl. Web when drawing lewis dot structures for ionic.
Ionic Bonds Are Caused By Electrons Transferring From One Atom To Another.
The example is for the nitrate ion. Web draw lewis structures for ionic compounds. Since it is often possible to draw more than one valid lewis structure for a molecule or molecular ion, we will need to assess which ones are more plausible or make better chemical sense. Web this chemistry video explains how to draw the lewis structures of ionic compounds.
Web A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.the diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. This involves crossing down the charges. Web the strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions.
We Can Draw The Lewis Structure Of Any Covalent Molecule By Following The Six Steps Discussed Earlier.
How to draw lewis structures. In this case, we can condense the last few steps, since not all of them apply. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. We will also go over what the octet rule is and all the e.
Six Electrons Are Used, And 6 Are Left Over.
When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Web here are the steps to draw a lewis structure. Since hydrogen is in group i it has one (1) valence electron in its shell. Web ionic lewis dot structures.