How To Draw Resonance Hybrids
How To Draw Resonance Hybrids - We say that the best or actual distribution (most stable) is a resonance hybrid of the contributing lewis representations. Craig beals shares his set of rules for drawing lewis structures of molecules that have resonance hybrids in chemistry. We just have to draw the resonance contributors in some order that makes sense. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an alkane is >2), chemists expected their structural formulas would contain a large number of double or triple bonds. Use curved arrows to represent electron movement. Understand the rules of resonance and identify where electrons can flow to or away from. Let’s see if we can correlate these drawing conventions to a valence bond theory picture of the bonding in a carboxylate group. Resonance contributors and the resonance hybrid. Curved arrows communicate electron flow (movement) Since double bonds are easily cleaved by oxidative.
Web this organic chemistry video tutorial explains how to draw resonance structures, how to identify the major resonance contributor, and how to draw the resonance hybrid. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an alkane is >2), chemists expected their structural formulas would contain a large number of double or triple bonds. Use curved arrows to represent electron movement. The actual structure isn't part of the time one structure and part of the time another structure. Draw curved arrows to show the movement of electrons. Determine the relative stability of resonance structures using a set of rules. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Web it should be noted, each individual resonance structure is averaged into a resonance hybrid which is both the true shape of the molecule and the most stable resonance form. Draw the lewis structure & resonance. Web however, sometimes benzene will be drawn with a circle inside the hexagon, either solid or dashed, as a way of drawing a resonance hybrid.
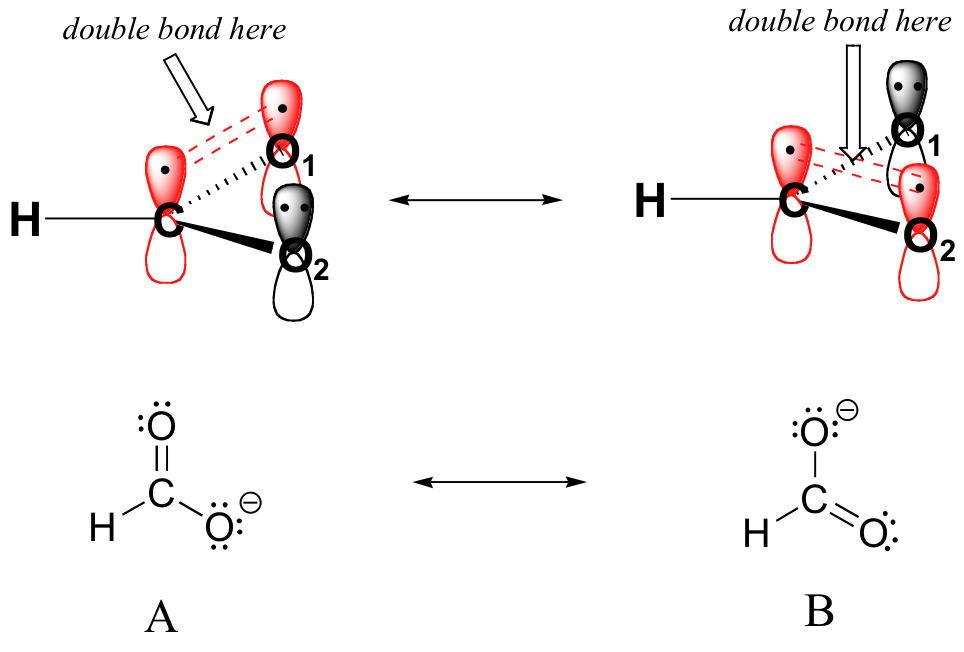
That lone pair is participating in resonance, which makes this nitrogen sp two hybridized, so it has a p orbital. Click to get pearson+ app download the mobile app. Confirm that no rules of. Web the net sum of valid resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule. Draw the lewis structure & resonance. Web resonance arises when more than one valid lewis structure can be drawn for a molecule or ion. Let’s see if we can correlate these drawing conventions to a valence bond theory picture of the bonding in a carboxylate group. Get a 10 bullets summary of the topic. This general chemistry video tutorial provides a basic introduction into resonance structures. A molecule that has several resonance structures is more stable than one with fewer.
14.4 The Resonance Hybrid Chemistry LibreTexts
Draw curved arrows to show the movement of electrons. Web it should be noted, each individual resonance structure is averaged into a resonance hybrid which is both the true shape of the molecule and the most stable resonance form. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). A molecule that has several resonance structures.
How to Draw Resonance Structures Organic Chemistry
830k views 6 years ago new ap & general chemistry video playlist. A molecule that has several resonance structures is more stable than one with fewer. Web resonance arises when more than one valid lewis structure can be drawn for a molecule or ion. Since double bonds are easily cleaved by oxidative. The structure is at all times a single.
[Solved] Chemistry Question. 2.29 Draw a resonance hybrid for each of
The general approach is described below: Rules for drawing contributing structures. Draw curved arrows to show the movement of electrons. Web when there is more than one reasonable way to draw a lewis representation, the true electron distribution is a compromise that has some of the characteristics of each of the reasonable structures. Web learn how to start your resonance.
Drawing Resonance Hybrids A\L Chemistry YouTube
Web it is useful to combine the resonance structures into a single structure called the resonance hybrid that describes the bonding of the molecule. Add only the lone pairs found on all resonance structures. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an alkane is >2), chemists expected their structural formulas.
Resonance Structures, Basic Introduction How To Draw The Resonance
Use the concept of resonance to explain structural features of molecules and ions. Draw curved arrows to show the movement of electrons. Since double bonds are easily cleaved by oxidative. The actual structure isn't part of the time one structure and part of the time another structure. The structure is at all times a single resonance hybrid of all the.
Intro to Resonance In Organic Chemistry Master Organic Chemistry
A molecule that has several resonance structures is more stable than one with fewer. Showing 1 of 10 videos. The overall electronic structure of the molecule or ion is given by the weighted average of these resonance structures and is referred to as the resonance hybrid. When drawing resonance structures, here are a few things you want to keep in.
How to Draw Resonance Contributors MCC Organic Chemistry
Web © 2024 google llc. Get a 10 bullets summary of the topic. Web the net sum of valid resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule. The overall electronic structure of the molecule or ion is given by the weighted average of these resonance structures and is referred to.
[40+] Resonance Hybrid Definition Chemistry
That lone pair is participating in resonance, which makes this nitrogen sp two hybridized, so it has a p orbital. Web it should be noted, each individual resonance structure is averaged into a resonance hybrid which is both the true shape of the molecule and the most stable resonance form. Understand the rules of resonance and identify where electrons can.
How to Draw Resonance Hybrid GOC electron displacement effect
Web 3 min read. When drawing resonance structures, here are a few things you want to keep in mind: Get a 10 bullets summary of the topic. Web draw the resonance structures of molecules or ions that exhibit delocalization. Web it should be noted, each individual resonance structure is averaged into a resonance hybrid which is both the true shape.
[Solved] Chemistry Question. 2.29 Draw a resonance hybrid for each of
Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Rules for drawing contributing structures. 830k views 6 years ago new ap & general chemistry video playlist. Add only the lone pairs found on all resonance structures. These structures are called resonance structures or contributing structures.
Curved Arrows Communicate Electron Flow (Movement)
These structures are called resonance structures or contributing structures. This organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an alkane is >2), chemists expected their structural formulas would contain a large number of double or triple bonds. Understand the rules of resonance and identify where electrons can flow to or away from.
Web However, Sometimes Benzene Will Be Drawn With A Circle Inside The Hexagon, Either Solid Or Dashed, As A Way Of Drawing A Resonance Hybrid.
Want to join the conversation? Draw curved arrows to show the movement of electrons. The structure is at all times a single resonance hybrid of all the structures. Web the net sum of valid resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule.
Showing 1 Of 10 Videos.
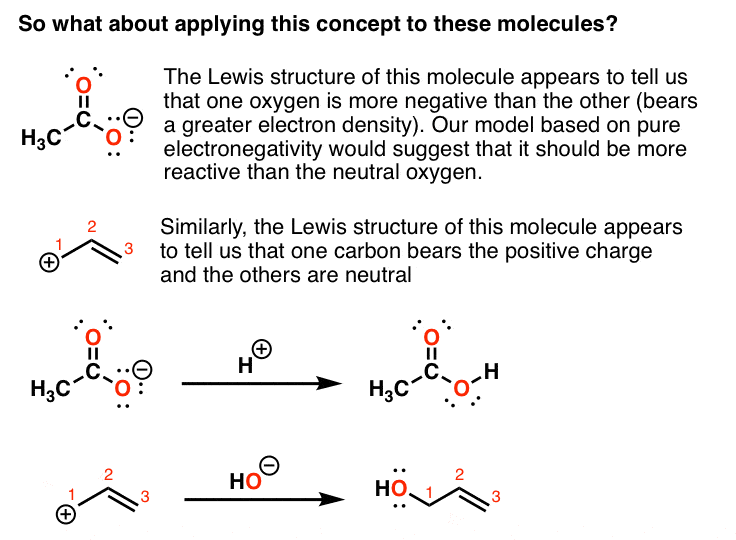
That lone pair is participating in resonance, which makes this nitrogen sp two hybridized, so it has a p orbital. Resonance is used to represent all the different ways that identical molecules can distribute electrons. Resonance contributors and the resonance hybrid. Some resonance structures are more favorable than others.
Web And So, Here's A Situation Where Drawing A Resonance Structure Helps Clue Us Into What's Actually Happening:
Use the concept of resonance to explain structural features of molecules and ions. Confirm that no rules of. It explains how to identify the major resonance contr. Web when there is more than one reasonable way to draw a lewis representation, the true electron distribution is a compromise that has some of the characteristics of each of the reasonable structures.




![[40+] Resonance Hybrid Definition Chemistry](https://cdn.masterorganicchemistry.com/wp-content/uploads/2019/11/2-summary-of-resonance-acetate-ion-resonance-forms-allyl-cation-resonance-hybrid.gif)
