How To Draw The Conjugate Base Of An Acid
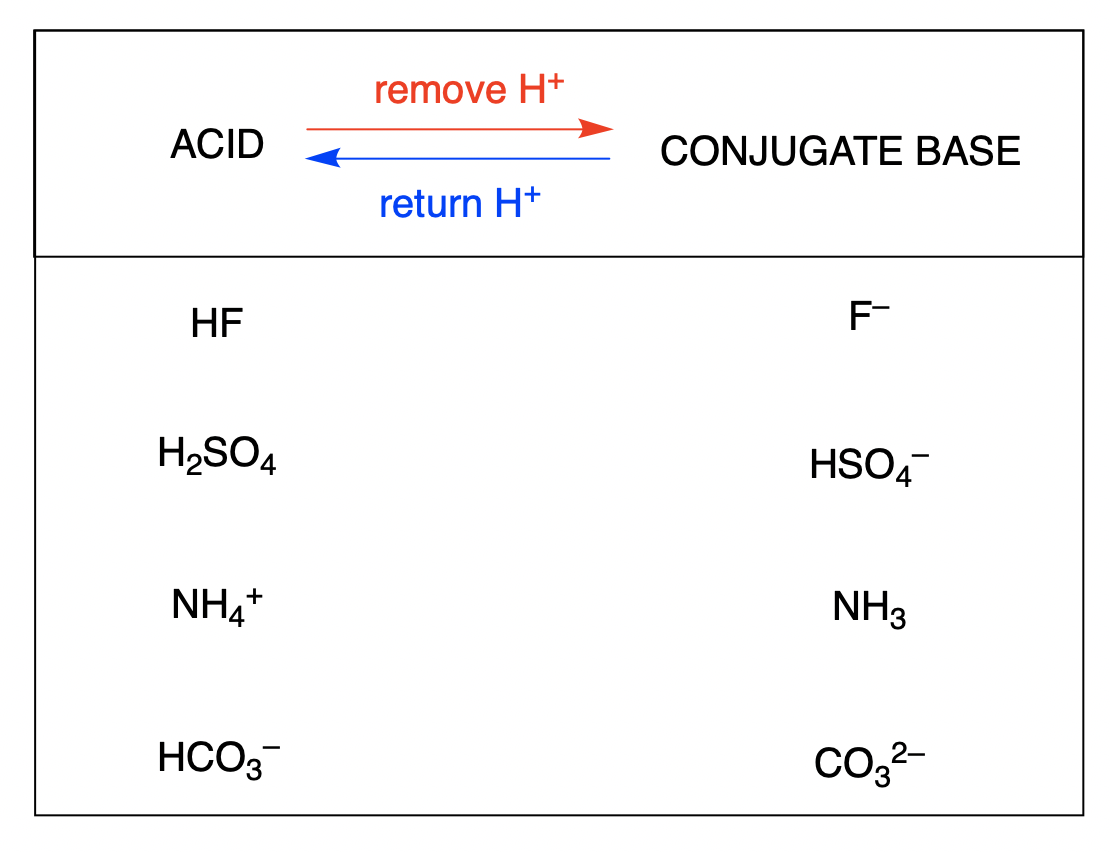
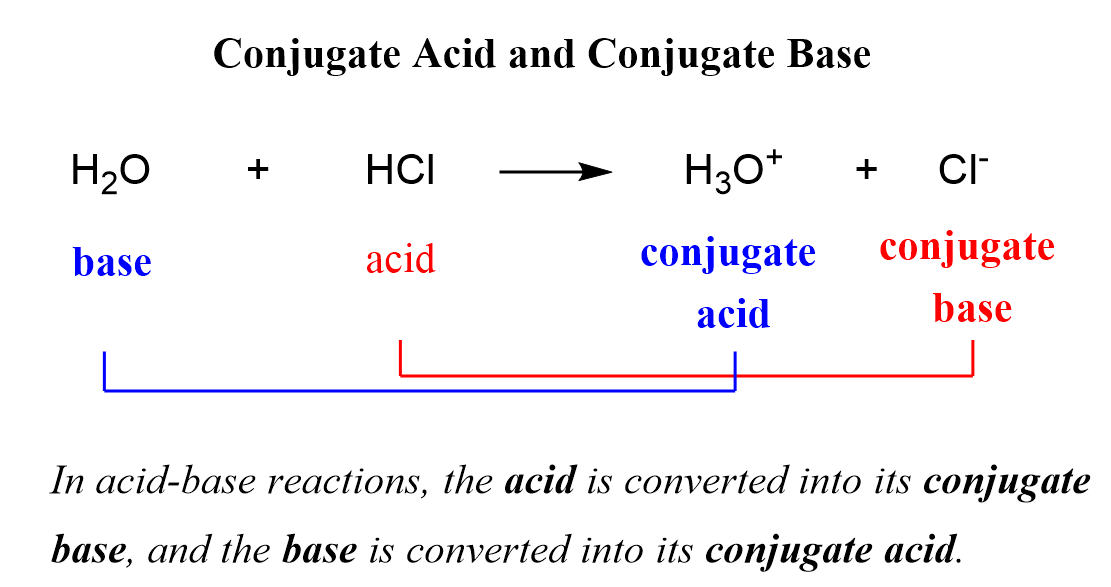
How To Draw The Conjugate Base Of An Acid - Ch 3 nh 2 is an amine and therefore a. The species formed from an acid when it donates a proton to a base. So that would be 10 to the second power, or 100 times more acidic. So h₂co₃ is the conjugate acid of hco₃⁻. Web this is two ph units, from 4.8 to 2.9 is pretty close to two ph units. The species formed from a base when it accepts a proton from an acid. Hcl is a strong acid. Web base + acid → conj a + conj b. There are two acids and two bases in. If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have a.
(b) ch 3 nh 2; The species formed from a base when it accepts a proton from an acid. When an acid donates a proton, it forms its conjugate base; There are two acids and two bases in. The species formed from an acid when it donates a proton to a base. Web this is two ph units, from 4.8 to 2.9 is pretty close to two ph units. Web so on the right, this right here must be the conjugate acid. We see that hco₃⁻ becomes h₂co₃. So h₂co₃ is the conjugate acid of hco₃⁻. Web let's draw the conjugate base for acetic acid.
We see that hco₃⁻ becomes h₂co₃. So h₂co₃ is the conjugate acid of hco₃⁻. Web this is two ph units, from 4.8 to 2.9 is pretty close to two ph units. Web let's draw the conjugate base for acetic acid. The species formed from an acid when it donates a proton to a base. We also practice writing formulas for conjugate acids and conjugate bases. Web so on the right, this right here must be the conjugate acid. If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have a. The species formed from a base when it accepts a proton from an acid. When an acid donates a proton, it forms its conjugate base;
Write the Formula for the Conjugate Acid of Each Base
If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have a. When a base accepts a proton, it forms its conjugate acid. We see that hco₃⁻ becomes h₂co₃. (b) ch 3 nh 2; The removal of cr(vi) at a.
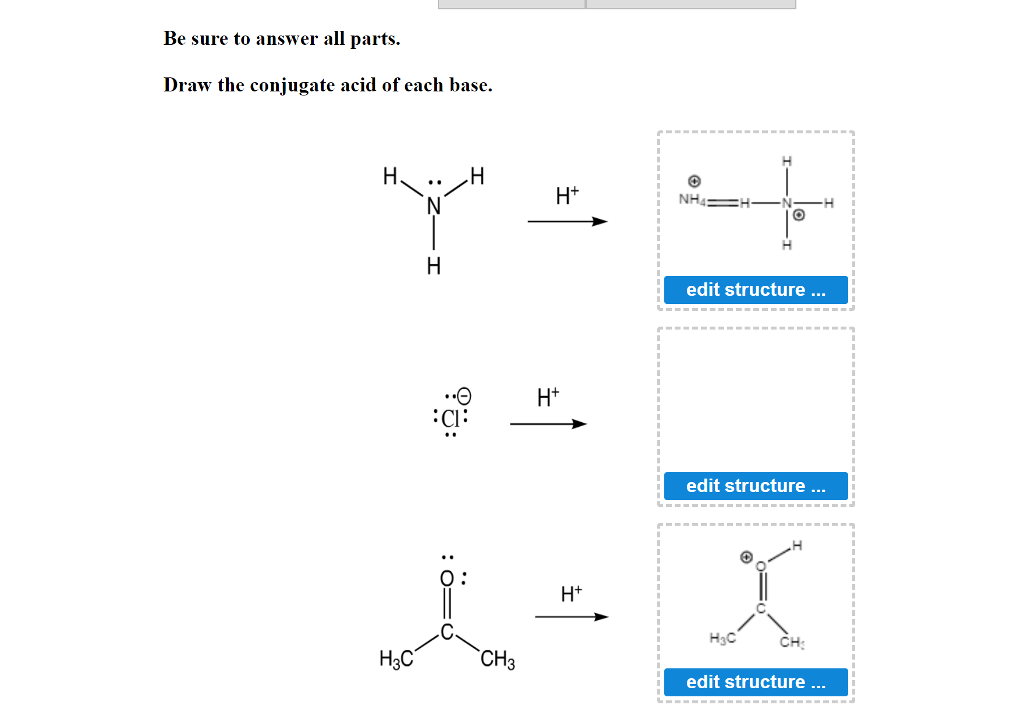
Solved Be sure to answer all parts. Draw the conjugate acid
The species formed from an acid when it donates a proton to a base. So this is the conjugate acid on the right. Web so on the right, this right here must be the conjugate acid. Ch 3 nh 2 is an amine and therefore a. So h₂co₃ is the conjugate acid of hco₃⁻.
Trick to Find Conjugate Acid and Conjugate Base Ionic Equilibrium
When a base accepts a proton, it forms its conjugate acid. Hcl is a strong acid. There are two acids and two bases in. The species formed from an acid when it donates a proton to a base. We also practice writing formulas for conjugate acids and conjugate bases.
Acids, bases and salts Learning Lab
Web let's draw the conjugate base for acetic acid. The removal of cr(vi) at a. The species formed from a base when it accepts a proton from an acid. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. And so chloroacetic acid is much more.
Enter the Conjugate Base for Each Acid.
The removal of cr(vi) at a. Web let's draw the conjugate base for acetic acid. The species formed from a base when it accepts a proton from an acid. Hcl is a strong acid. Web so on the right, this right here must be the conjugate acid.
CHEM112 5 6 drawing conjugate acids and bases YouTube
We see that hco₃⁻ becomes h₂co₃. (b) ch 3 nh 2; Conjugate acid and conjugate base. So h₂co₃ is the conjugate acid of hco₃⁻. The species formed from a base when it accepts a proton from an acid.
How to typset/draw conjugate acids and bases
If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have a. The species formed from a base when it accepts a proton from an acid. The species formed from an acid when it donates a proton to a base. When an acid donates a proton, it forms.
Chapter 10 Exercises 3 and 4 Drawing Conjugate Acids and Conjugate
And so chloroacetic acid is much more. If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have a. Ch 3 nh 2 is an amine and therefore a. Conjugate acid and conjugate base. Web this is two ph units, from 4.8 to 2.9 is pretty close to.
Acids and Bases (Alevel) ChemistryStudent
Web let's draw the conjugate base for acetic acid. If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have a. Web what is the conjugate acid or the conjugate base of (a) hcl; So h₂co₃ is the conjugate acid of hco₃⁻. (b) ch 3 nh 2;
Conjugate Acid and Conjugate Base Chemistry Steps
We also practice writing formulas for conjugate acids and conjugate bases. Web what is the conjugate acid or the conjugate base of (a) hcl; The species formed from an acid when it donates a proton to a base. When an acid donates a proton, it forms its conjugate base; Conjugate acid and conjugate base.
The Removal Of Cr(Vi) At A.
(b) ch 3 nh 2; The species formed from a base when it accepts a proton from an acid. Hcl is a strong acid. Web so on the right, this right here must be the conjugate acid.
Web Base + Acid → Conj A + Conj B.
Conjugate acid and conjugate base. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. And so chloroacetic acid is much more. Web this is two ph units, from 4.8 to 2.9 is pretty close to two ph units.
We See That Hco₃⁻ Becomes H₂Co₃.
So that would be 10 to the second power, or 100 times more acidic. So h₂co₃ is the conjugate acid of hco₃⁻. When a base accepts a proton, it forms its conjugate acid. When an acid donates a proton, it forms its conjugate base;
Web Let's Draw The Conjugate Base For Acetic Acid.
We also practice writing formulas for conjugate acids and conjugate bases. There are two acids and two bases in. So this is the conjugate acid on the right. If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have a.