Hybridization Cheat Sheet
Hybridization Cheat Sheet - Web hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Hybridization can be classified as sp3,. Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1.
Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization can be classified as sp3,. Web hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has.
Web hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Hybridization can be classified as sp3,. Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1.
hybrid orbitals infographic. Linus Pauling's explanation of bonding
Web hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Hybridization can be classified as sp3,. Web a quick way to figure out the.
Hybridization Cheat Sheet
Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has. Web hybridization.
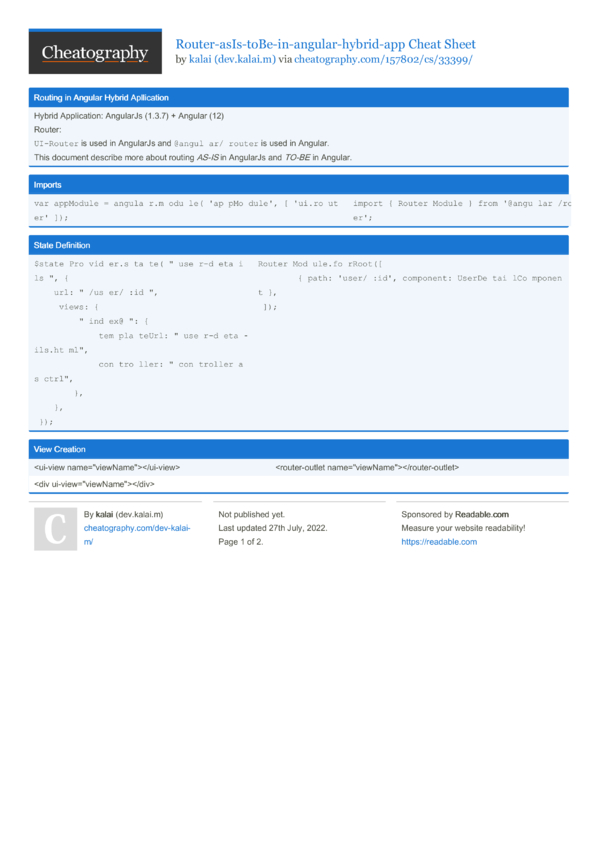
Orbital Hybridization Cheat Sheet Example Images
Web hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Web a quick way to figure out the hybridization of a specific atom in.
Hybridization Cheat Sheet
Hybridization can be classified as sp3,. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Web hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Web a quick way to figure out the.
Hybridization Cheat Sheet
Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Web hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Web a quick way to figure out the.
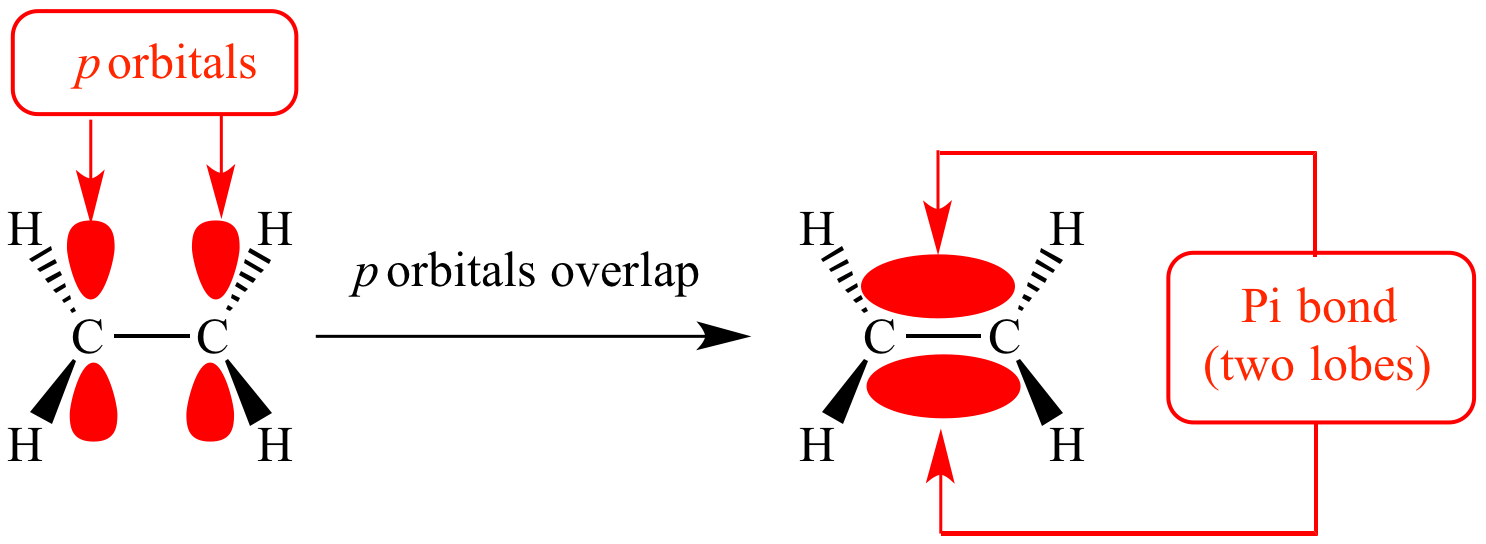
Carbon Hybridization chemistry
Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has. Web hybridization.
Machine Learning Algorithms Cheat Sheet — Accel.AI
Hybridization can be classified as sp3,. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in.
Orbital Hybridization Cheat Sheet Example Images
Hybridization can be classified as sp3,. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has. Web hybridization.
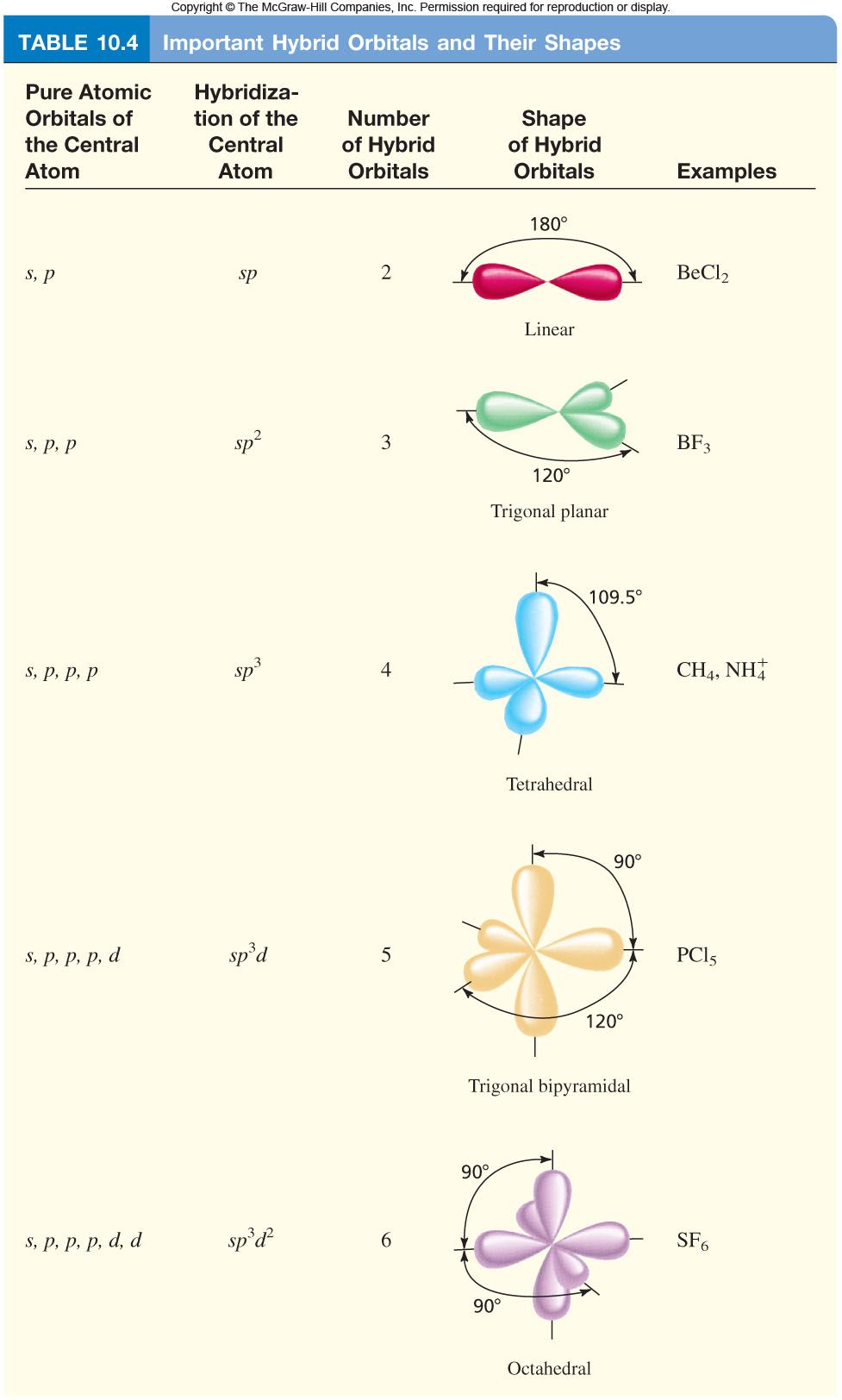
Orbital Hybridization "Cheat Sheet"? + Example
Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy. Hybridization can be classified as sp3,. Web here’s.
Hybridization Cheat Sheet
Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has. Hybridization can be classified as sp3,. Web hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. The phenomenon of mixing up of.
Web Hybridization Is The Concept Of Mixing Or Combining Of Two Atomic Orbitals To Create A New Type Of Hybridized Orbitals.
Web a quick way to figure out the hybridization of a specific atom in a molecule (central atoms) is by the number of bonds that atom has. Hybridization can be classified as sp3,. Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. The phenomenon of mixing up of atomic orbitals of similar energies and formation of equivalent number of entirely new orbitals of identical shape and energy.