Iron Reacts With Oxygen To Form Rust Physical Or Chemical
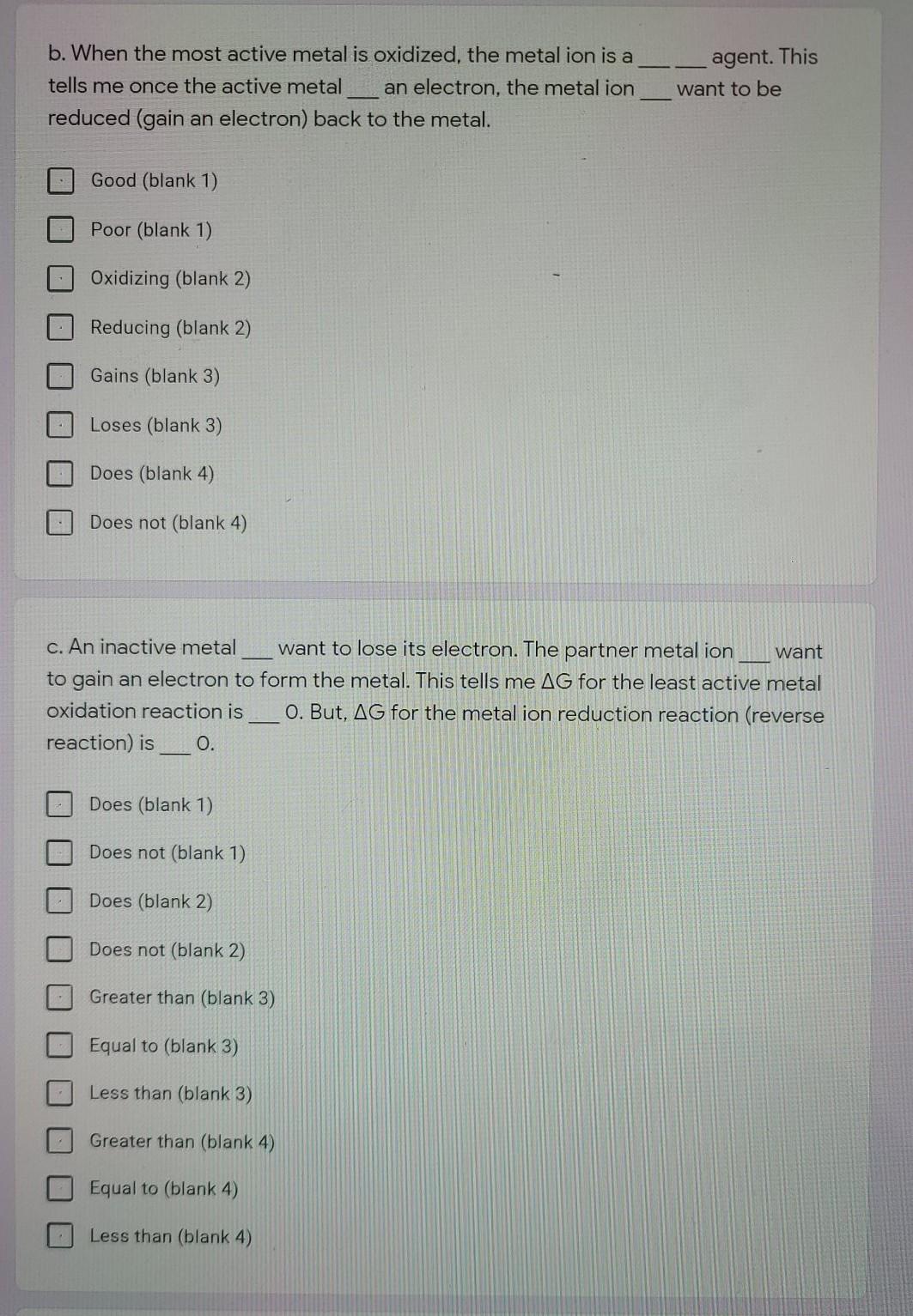
Iron Reacts With Oxygen To Form Rust Physical Or Chemical - Web iron and oxygen react together and form rust. Iron + oxygen + water → hydrated iron(iii) oxide When iron comes into contact with oxygen and water, ferric oxide, sometimes known as rust, forms. Write a balanced equation for this reaction. List the reactants and products of this reaction. Web iron combusts in oxygen to form various iron oxides, mainly iron (iii) oxide: Rusting is a chemical reaction (oxidation) not a property. Web 10/20/2020 chemistry high school verified answered • expert verified iron reacts with oxygen to form iron oxide (rust). 4fe (s) + 3o2 (g) (arrow. Which of the following explains the change?
Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Which best describes this statement? The rusting of iron is a chemical reaction of iron with oxygen in the air to form iron (iii) oxide. Upon reacting with oxygen, iron will be oxidized to either the +3. Here is the word equation for the reaction: Web is iron rusting in the presence of oxygen a chemical or physical property? Although rust may generally be termed as oxidation, that term is much more general and descri… Iron + oxygen + water → hydrated iron(iii) oxide The rusting of iron is a chemical reaction of iron with oxygen in the air to. Write a balanced equation for this reaction.
Which of the following explains the change? This hydrated iron (ill) oxide is. Web during rusting, iron combines with oxygen in the air in the presence of water to generate fe 2 o 3.xh 2 o, a hydrated iron (iii) oxide. Iron reacts with molecular oxygen to form rust, or iron (iii) oxide in the following balanced equation: Write a balanced equation for this reaction. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Web is iron rusting in the presence of oxygen a chemical or physical property? Web the reaction between iron and oxygen to form rust occurs spontaneously. List the reactants and products of this reaction. Web if you're wondering why this is, it's due to the fact that in chemical reactions, physical appearance of the products changes compared to the reactants.
Corrosion Rusting Of Iron, Rust Prevention & Preventive measures
Here is the word equation for the reaction: Is a specific example of corrosion, which occurs when iron or steel reacts with oxygen and water: Rust results in the formation of metal oxide. Iron + oxygen + water → hydrated iron(iii) oxide The balanced chemical reaction can be represented.
Rust is Caused by a Reaction between Oxygen and Iron. Stock Photo
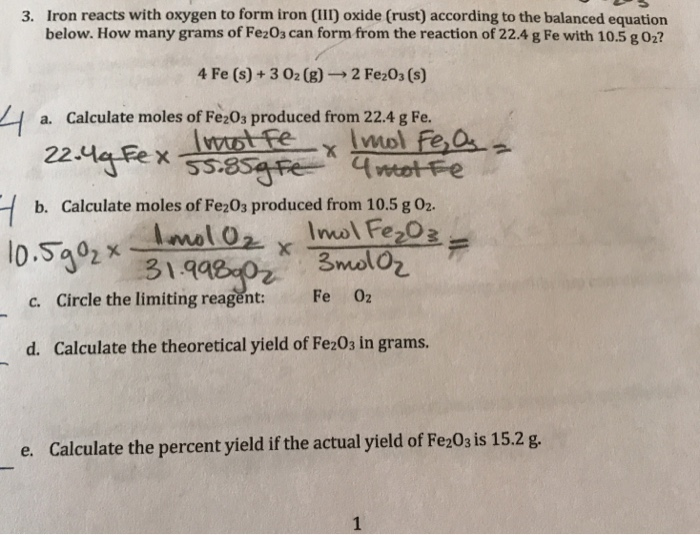
The rusting of iron is a chemical reaction of iron with oxygen in the air to. Web when iron reacts with atmospheric oxygen during rusting, it loses three electrons and becomes oxidized. Over time, the oxygen combines with the metal forming new compounds collectively called rust. Is a specific example of corrosion, which occurs when iron or steel reacts with.
Rust is Caused by a Reaction between Oxygen and Iron. Stock Photo
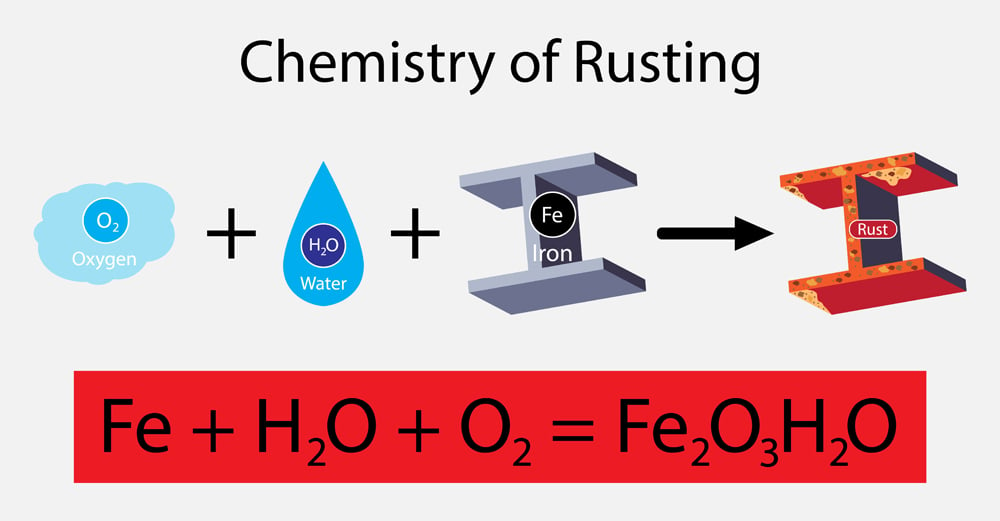
4fe (s) + 3o2 (g) (arrow. Rust results in the formation of metal oxide. The rusting of iron is a chemical reaction of iron with oxygen in the air to form iron (iii) oxide. 4 fe (s) + 3 o2 (g) ==> 2 fe2o3 (s) iron in its usual bulk solid form will only burn when in pure oxygen. Web.
Solved 1. Iron Reacts With Oxygen To Form Rust. A. Is The...
Web rust iron and oxygen combine to form iron oxide, or rust. Based on your experience, does this reaction occur rapidly? List the reactants and products of this reaction. When iron comes into contact with oxygen and water, ferric oxide, sometimes known as rust, forms. Iron + oxygen + water → hydrated iron(iii) oxide
PPT The Law of Conservation of Matter PowerPoint Presentation ID
Web when iron reacts with atmospheric oxygen during rusting, it loses three electrons and becomes oxidized. Web is iron rusting in the presence of oxygen a chemical or physical property? Iron + oxygen + water → hydrated iron(iii) oxide The rusting of iron is a chemical reaction of iron with oxygen in the air to. Rust is a general name.
Single Replacement Reaction vs Double Replacement Reaction Differences
Web is iron rusting in the presence of oxygen a chemical or physical property? Web if you're wondering why this is, it's due to the fact that in chemical reactions, physical appearance of the products changes compared to the reactants. Iron + water + oxygen → hydrated iron. Web rust iron and oxygen combine to form iron oxide, or rust..
Rust is the oxide of iron Rust is formed due to reaction of iron with
Web 3.) in balancing problems, i like to always start with oxygen. Upon reacting with oxygen, iron will be oxidized to either the +3. Iron is a gray metal and oxygen is a colorless gas. Web the reaction between iron and oxygen to form rust occurs spontaneously. Web iron and oxygen react together and form rust.
Solved 3. Iron reacts with oxygen to form iron (III) oxide
4fe (s) + 3o2 (g) (arrow. Web the reaction between iron and oxygen to form rust occurs spontaneously. List the reactants and products of this reaction. Iron reacts with molecular oxygen to form rust, or iron (iii) oxide in the following balanced equation: Rusting is a chemical reaction (oxidation) not a property.
What Is Oxidation? What Is An Oxidation Number?
Here is the word equation for the reaction: Based on your experience, does this reaction occur rapidly? Web is iron rusting in the presence of oxygen a chemical or physical property? Web the reaction between iron and oxygen to form rust occurs spontaneously. Web when iron reacts with atmospheric oxygen during rusting, it loses three electrons and becomes oxidized.
science chemistry chemical reaction iron oxide Fundamental
Iron is a gray metal and oxygen is a colorless gas. Iron + oxygen + water → hydrated iron(iii) oxide The balanced chemical reaction can be represented. Write a balanced equation for this reaction. Although rust may generally be termed as oxidation, that term is much more general and descri…
Web 3.) In Balancing Problems, I Like To Always Start With Oxygen.
The rusting of iron is a chemical reaction of iron with oxygen in the air to form iron (iii) oxide. Iron + water + oxygen → hydrated iron. Web the reaction between iron and oxygen to form rust occurs spontaneously. The balanced chemical reaction can be represented.
Which Of The Following Explains The Change?
Web during rusting, iron combines with oxygen in the air in the presence of water to generate fe 2 o 3.xh 2 o, a hydrated iron (iii) oxide. 4 fe (s) + 3 o2 (g) ==> 2 fe2o3 (s) iron in its usual bulk solid form will only burn when in pure oxygen. Rust results in the formation of metal oxide. Web iron and oxygen react together and form rust.
Web If You're Wondering Why This Is, It's Due To The Fact That In Chemical Reactions, Physical Appearance Of The Products Changes Compared To The Reactants.
Which best describes this statement? Iron reacts with molecular oxygen to form rust, or iron (iii) oxide in the following balanced equation: When iron comes into contact with oxygen and water, ferric oxide, sometimes known as rust, forms. Iron is a gray metal and oxygen is a colorless gas.
Web 10/20/2020 Chemistry High School Verified Answered • Expert Verified Iron Reacts With Oxygen To Form Iron Oxide (Rust).
Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Write a balanced equation for this reaction. Web iron combusts in oxygen to form various iron oxides, mainly iron (iii) oxide: List the reactants and products of this reaction.