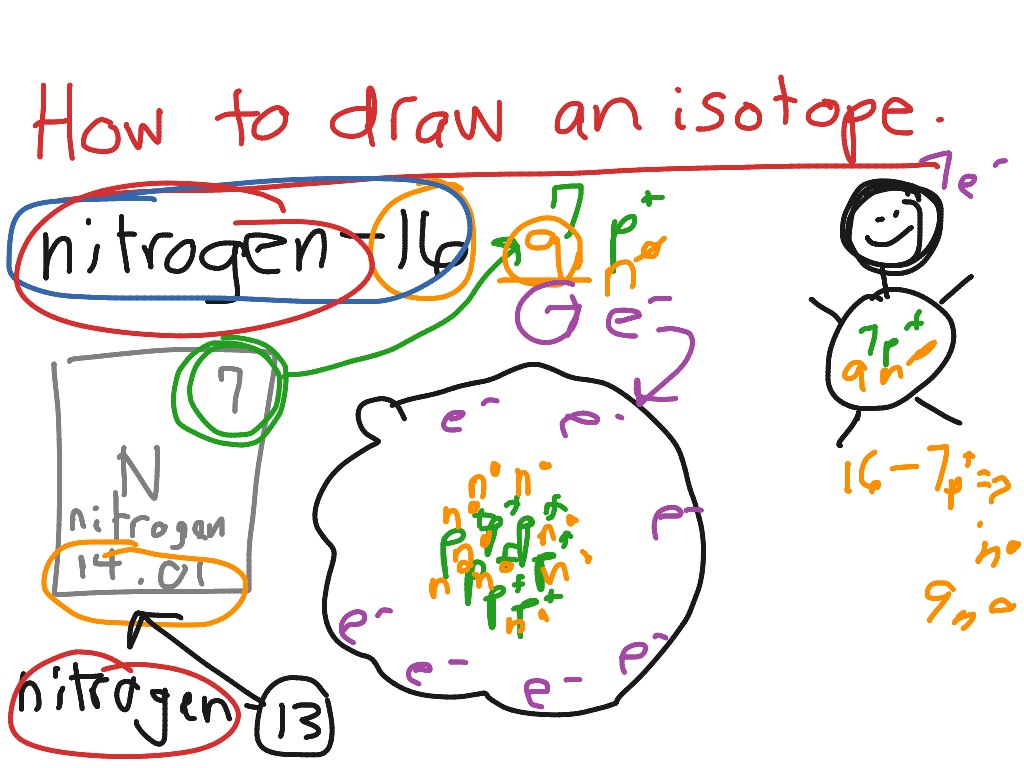
Isotope Drawing
Isotope Drawing - Identify the element and write symbols for the isotopes. Web how to draw the atomic structure of atoms. There are three scaffolded activities. Number of protons and neutrons. Manipulate the atomic weight equation to calculate various unknown variables. Distinguish between atomic weight, atomic number, and mass number. Web this online quiz is intended to give you extra practice in writing names and notation for hundreds of elements and isotopes. Why is isotope notation important? Web learn and interactive with isotopes. Use red ink for protons and black ink for electrons.
Identify the element and write symbols for the isotopes. Learn how to identify different isotopes of an element, how to calculate the average atomic mass from isotopic abundance, and how to compare the properties of isotopes. The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element. Distinguish between atomic weight, atomic number, and mass number. Web learn and interactive with isotopes. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Why is isotope notation important? These standards provide guidelines to assist the council in applying those criteria in iowa code sections 135.64(1) a to r and 135.64(3). Web the isotopes concept builder challenges learners to use information regarding the number of protons, neutrons, and electrons within an atom in order to create the isotope notation for that atom. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
Why is isotope notation important? It can also plot either calculated or manually inputted isotopic distributions. Web the isotopes concept builder challenges learners to use information regarding the number of protons, neutrons, and electrons within an atom in order to create the isotope notation for that atom. There are three scaffolded activities. Criteria which are measured by a standard are cited in parentheses following each standard. Calculate atomic weight from percent abundance. Learn how to identify different isotopes of an element, how to calculate the average atomic mass from isotopic abundance, and how to compare the properties of isotopes. These standards provide guidelines to assist the council in applying those criteria in iowa code sections 135.64(1) a to r and 135.64(3). Replicate the examples above, but in this exercise, have the students draw the structure. Web an element with three stable isotopes has 82 protons.
Isotopes and Isobars Definition, Uses and Difference Teachoo
Web an element with three stable isotopes has 82 protons. Web draw a bohr model of the isotope on the paper ; Number of protons and neutrons. Then play a game to test your ideas! Refer to the periodic table and use the number of protons to identify the element.
PreChemistry
Web isotope, one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behaviour but with different atomic masses and physical properties. This simulation is part of the phet project, which offers free math and science simulations for students and. Refer to the periodic.
What Is an Isotope? WorldAtlas
Web how to draw the atomic structure of atoms. Web interactive periodic table showing names, electrons, and oxidation states. Select your preferences below and click 'start' to give it a try! Learn how to identify different isotopes of an element, how to calculate the average atomic mass from isotopic abundance, and how to compare the properties of isotopes. Web about.
How to Draw an Isotope Science ShowMe
Every chemical element has one or more isotopes. Web how to draw the atomic structure of atoms. If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake. Isotopes are atoms of the same element that differ in the number of neutrons in their atomic nuclei..
Isotopes of carbon, illustration Stock Image C028/6464 Science
Then play a game to test your ideas! Web the isotopes concept builder challenges learners to use information regarding the number of protons, neutrons, and electrons within an atom in order to create the isotope notation for that atom. Web draw a bohr model of the isotope on the paper ; The number of protons and the mass number of.
How to Calculate Isotopes Sciencing
The separate isotopes contain 124, 125, and 126 neutrons. This simulation is part of the phet project, which offers free math and science simulations for students and. There are three naturally occurring isotopes of carbon: The number of protons and the mass number of an atom define the type of atom. Web the number of nucleons (both protons and neutrons).
DOE Explains...Isotopes Department of Energy
Then play a game to test your ideas! Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. This program can calculate the isotopic distribution of a given chemical species. Visualize trends, 3d orbitals, isotopes, and mix compounds. The number of protons and the mass number of an atom define the type.
Isotopes Definition and Examples in Chemistry
Distinguish between atomic weight, atomic number, and mass number. Calculate atomic weight from percent abundance. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Drawing atomic structure requires only a simple understanding of the components of atomic structure. There are three scaffolded activities.
Isotopes What are Isotopes? Relative Atomic Mass
Are stable, occurring in a natural proportion of approximately 93:1. Web how to draw the atomic structure of atoms. Web an element with three stable isotopes has 82 protons. Manipulate the atomic weight equation to calculate various unknown variables. Isotopes are atoms of the same element that differ in the number of neutrons in their atomic nuclei.
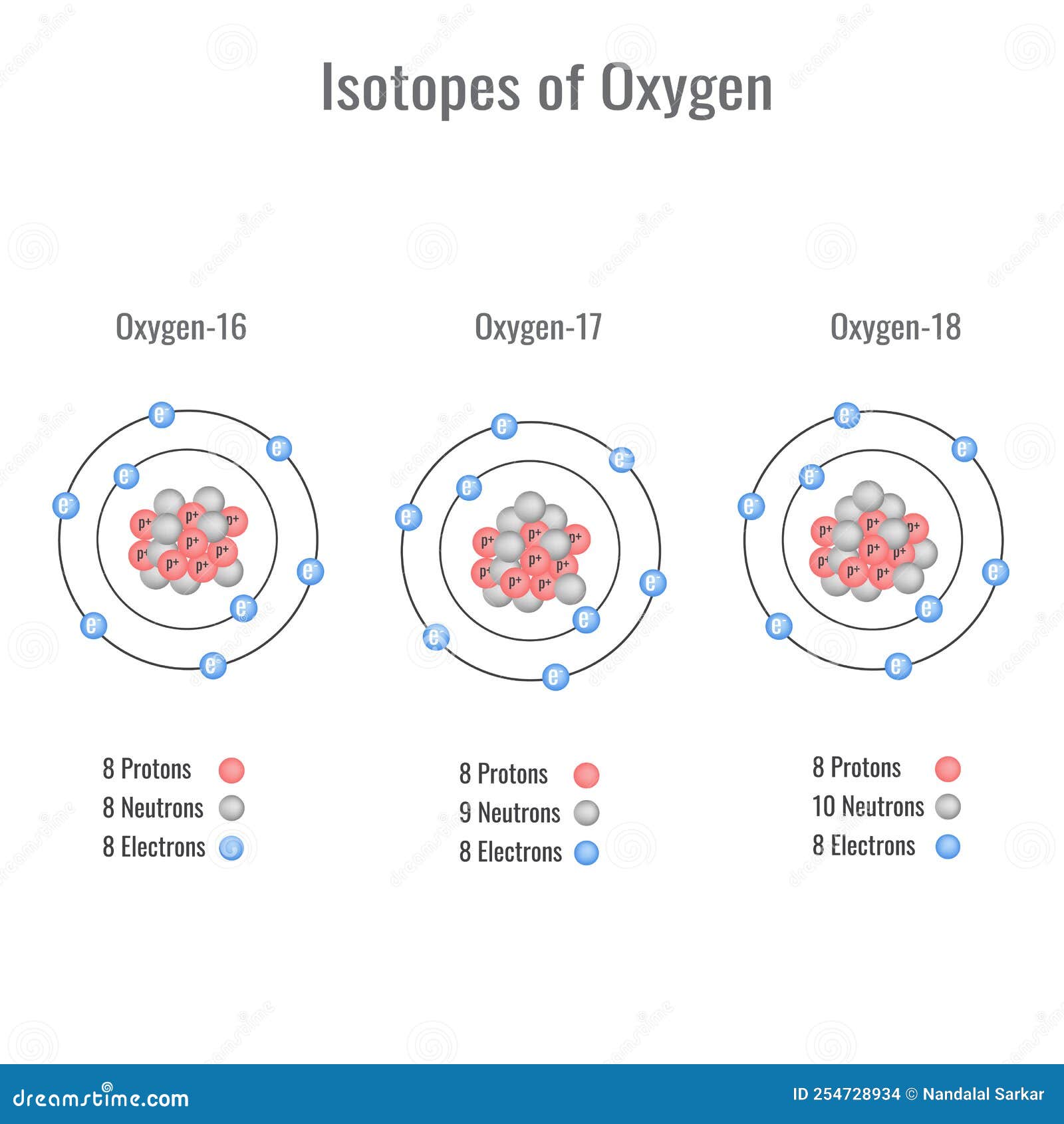
Isotopes of Oxygen Vector Illustration Stock Vector Illustration of
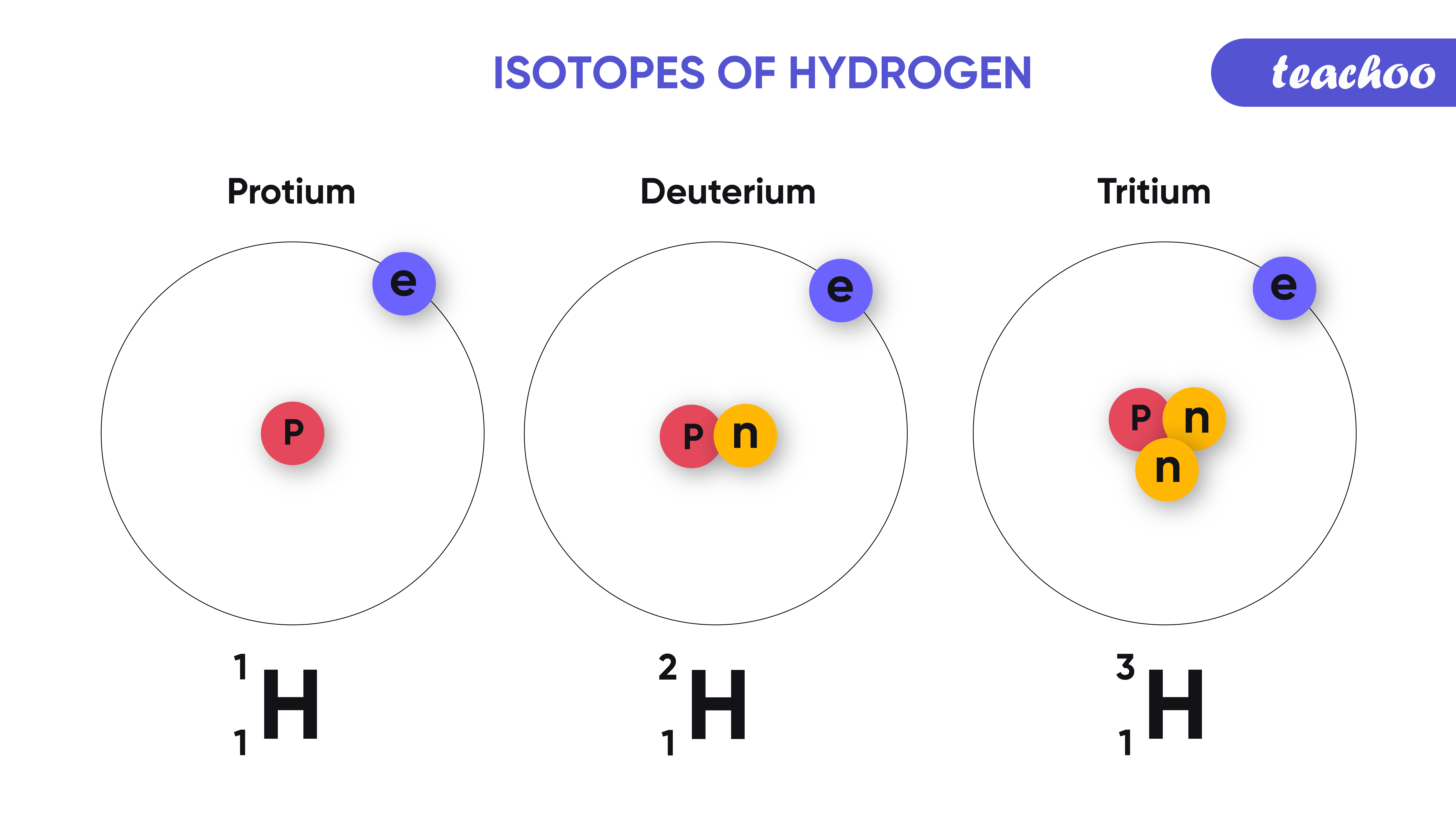
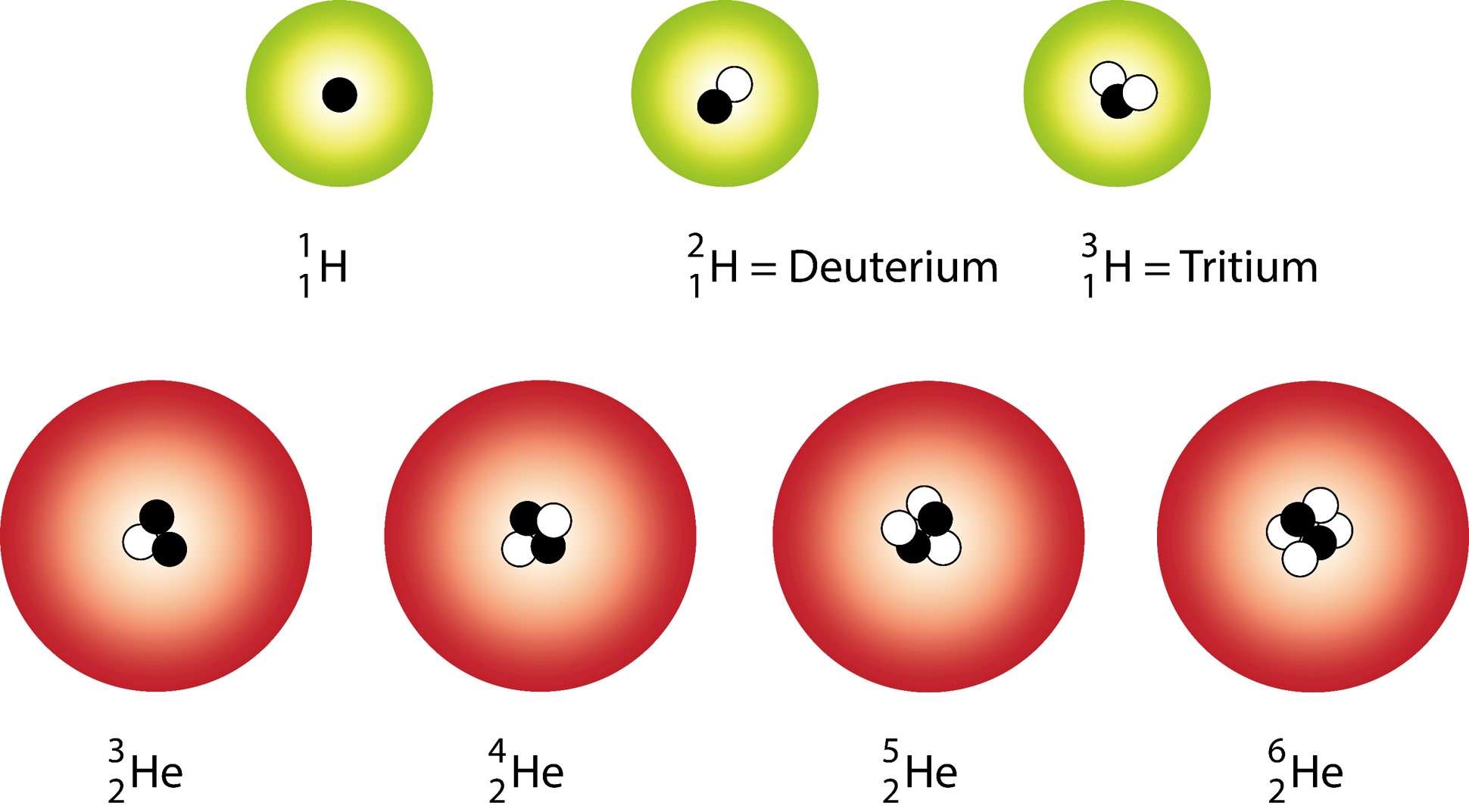
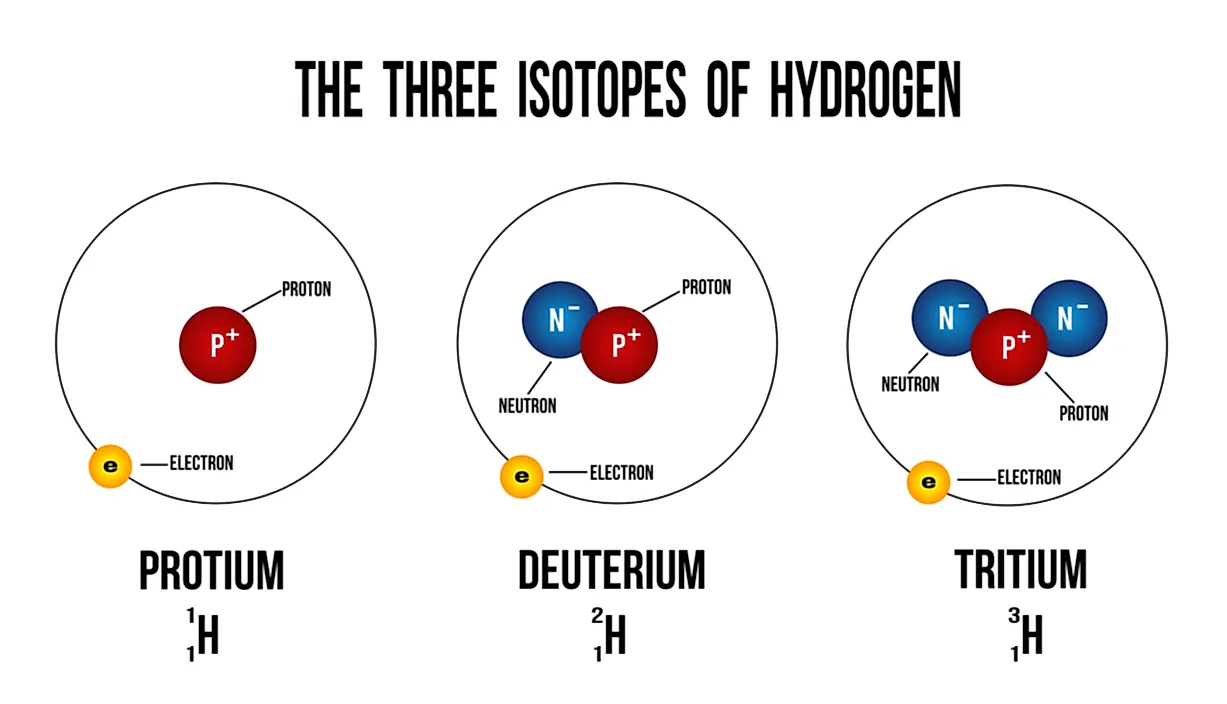
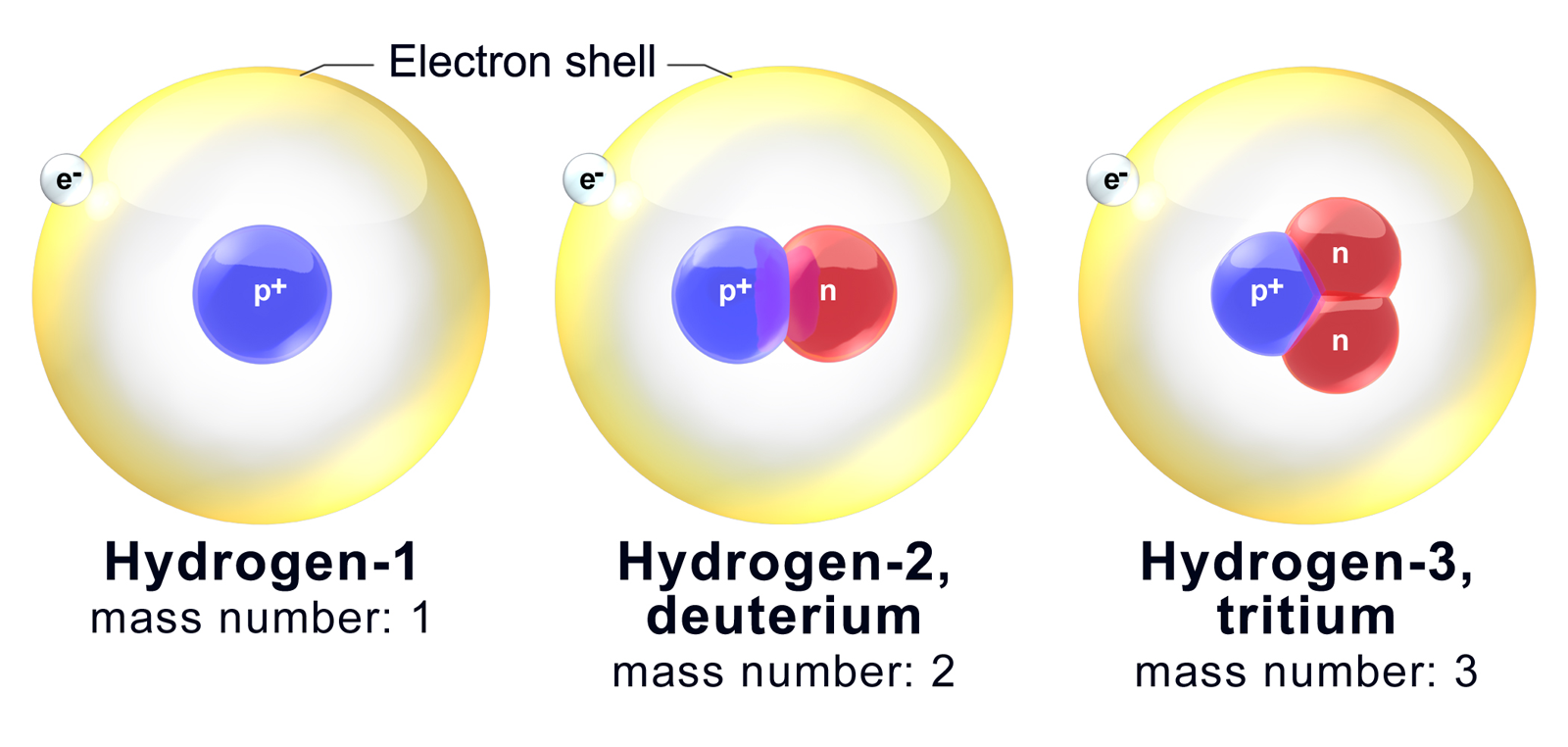
Deuterium is an isotope of hydrogen proton. The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element. Every chemical element has one or more isotopes. Web explore the concept of isotopes and atomic mass with this interactive simulation. Why is isotope notation important?
Drawing Atomic Structure Requires Only A Simple Understanding Of The Components Of Atomic Structure.
This program can calculate the isotopic distribution of a given chemical species. Select your preferences below and click 'start' to give it a try! Use the periodic table and bohr model to identify the name of the isotope Calculate atomic weight from percent abundance.
This Quiz Aligns With The Following Ngss Standard (S):
Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The atomic number of carbon is 6, which means. Why is isotope notation important? Isotopes have different atomic masses.
These Standards Provide Guidelines To Assist The Council In Applying Those Criteria In Iowa Code Sections 135.64(1) A To R And 135.64(3).
Web learn and interactive with isotopes. Then play a game to test your ideas! Web explore the concept of isotopes and atomic mass with this interactive simulation. Then play a game to test your ideas!
Replicate The Examples Above, But In This Exercise, Have The Students Draw The Structure.
Web the isotopes concept builder challenges learners to use information regarding the number of protons, neutrons, and electrons within an atom in order to create the isotope notation for that atom. Atoms of the same element with different mass numbers are called isotopes. Every chemical element has one or more isotopes. Number of protons and neutrons.


/Isotope-58dd6b415f9b5846830254ae.jpg)