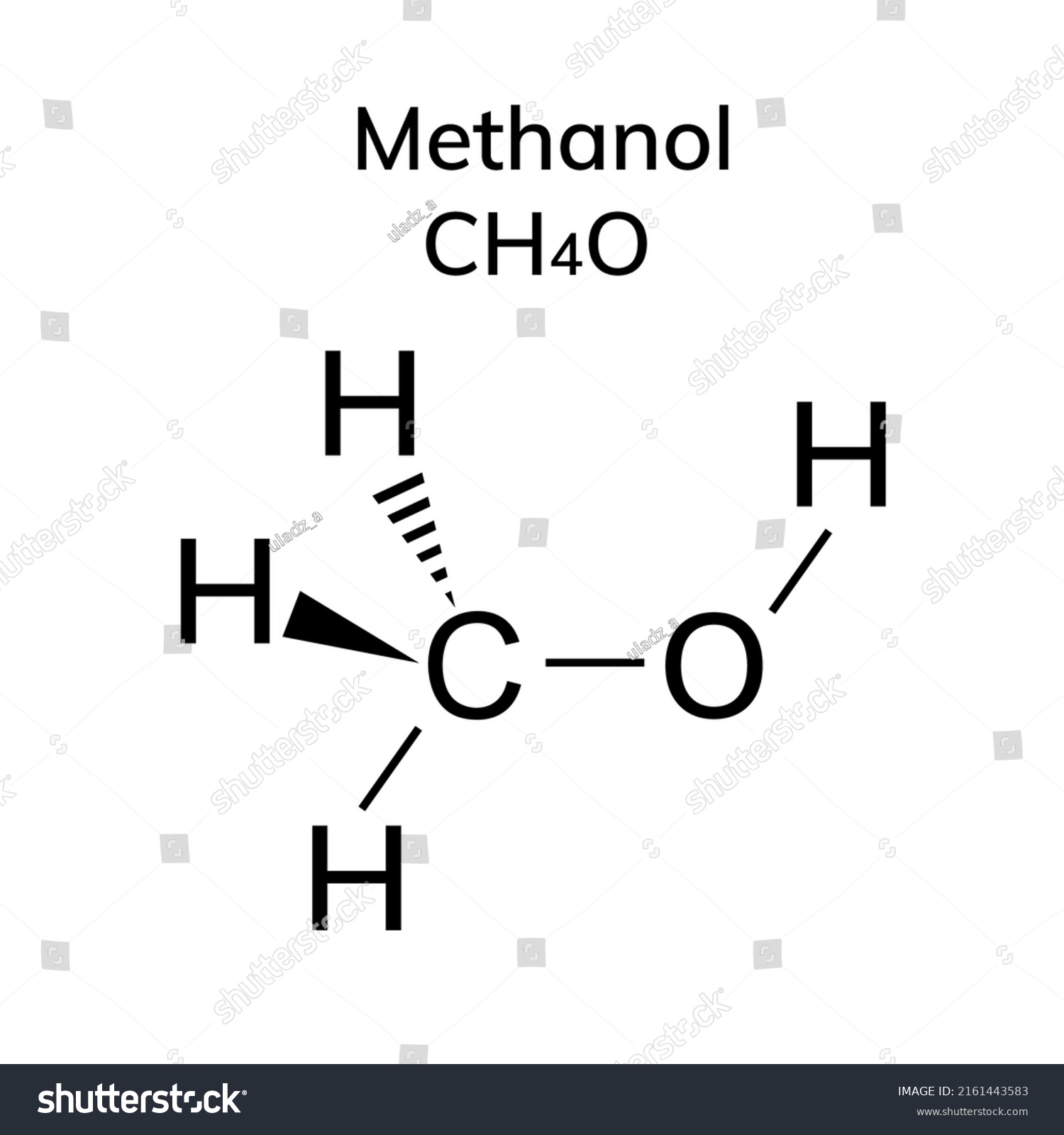
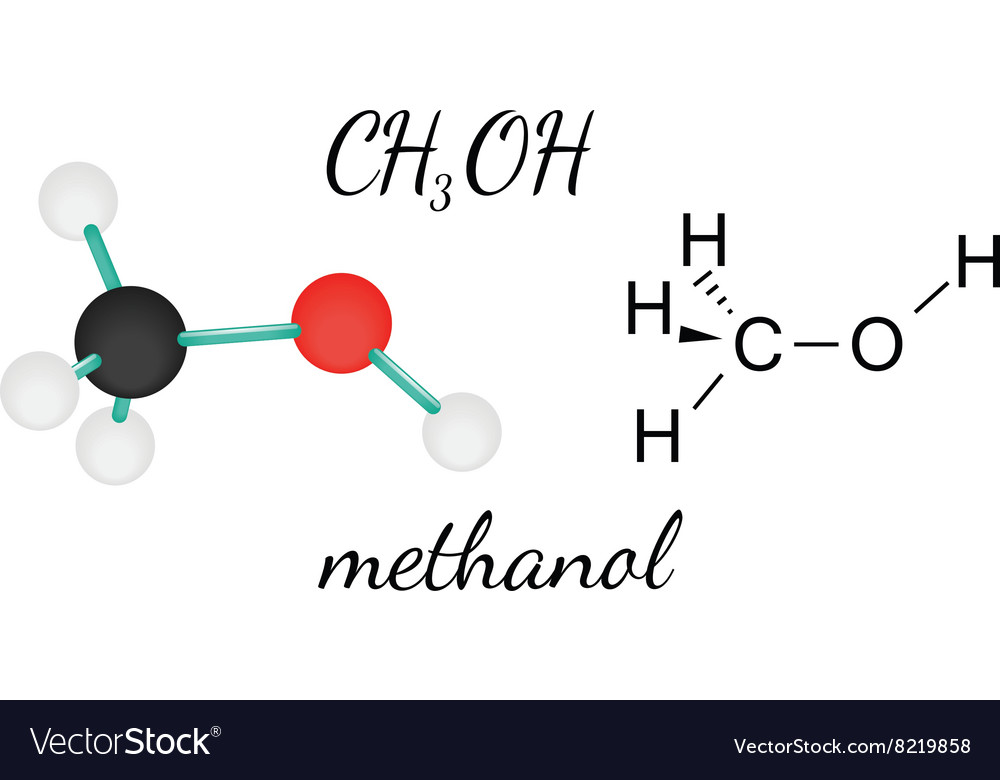
Methanol Structural Formula Drawing
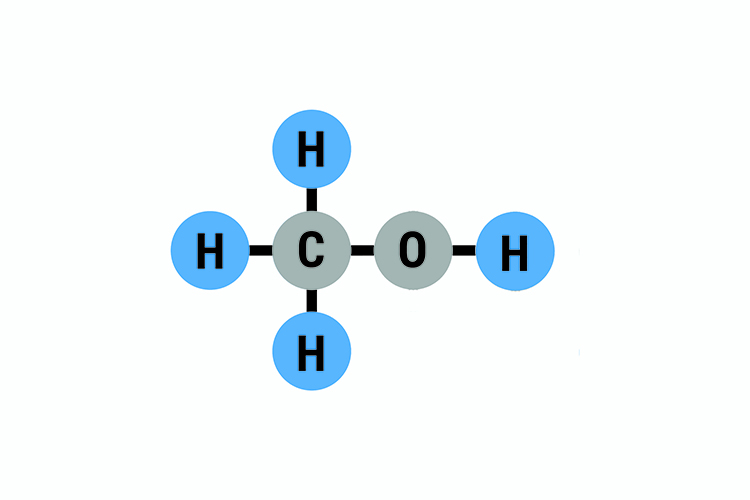
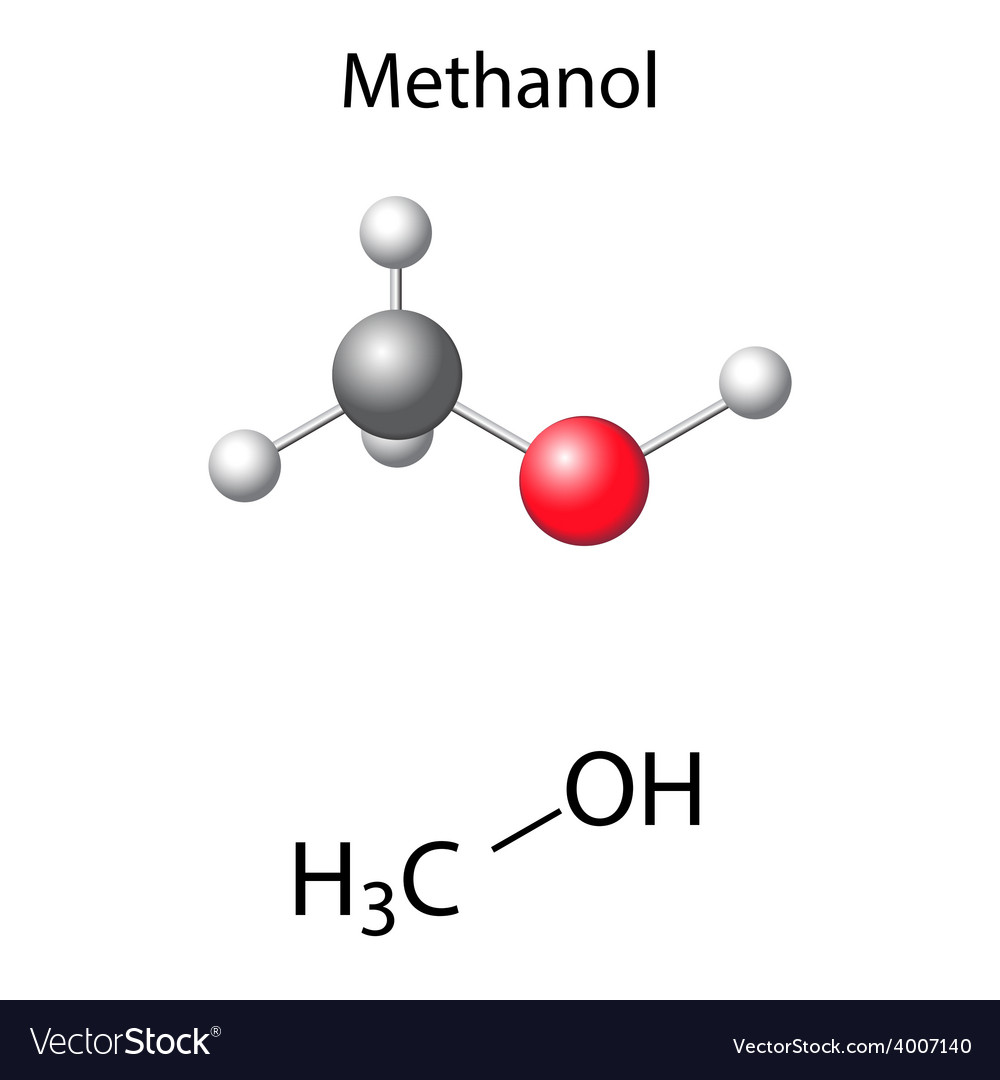
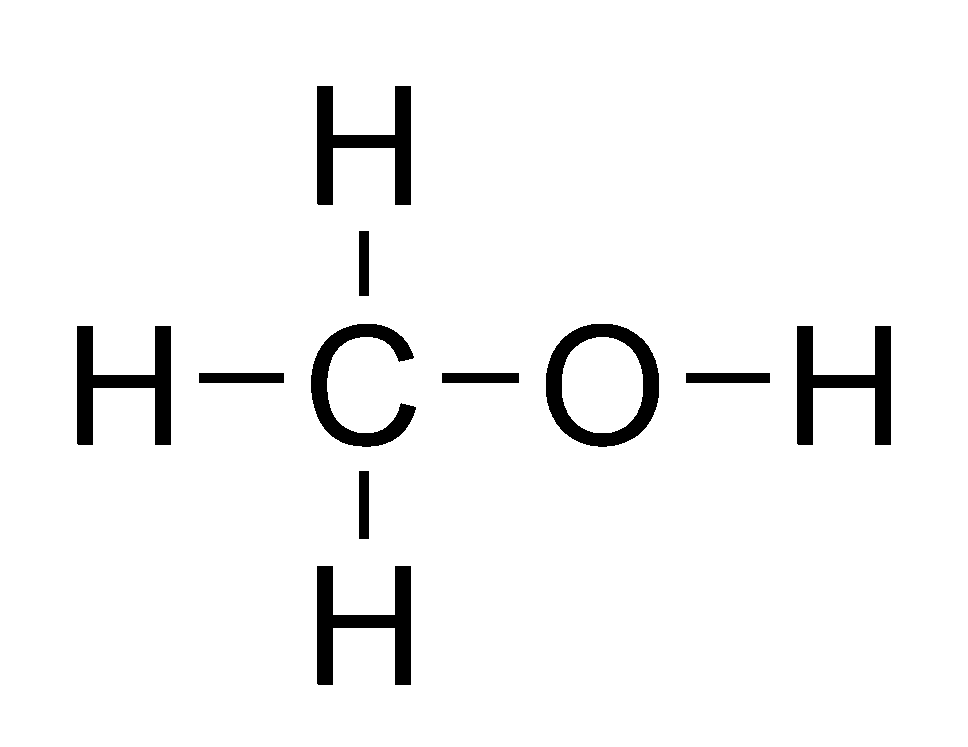
Methanol Structural Formula Drawing - Mark charges on atoms if there are charges. Mark lone pairs on atoms. Web organic chemistry ways to draw and represent molecules condensed structure. Total number of electrons of the valance shells of ch 3 oh. Condensed structural formula for ethanol: Writing methanol as ch 4 o tells nothing about its structure. In contrast, the structural formula (figure \(\pageindex{3b}\)) indicates how the atoms are connected, but it makes methanol look as if it is planar (which it is not). Formal charge on oxygen =. Web the lewis structure of ch3oh, also known as methanol, is a representation of the molecule’s bonding and electron distribution. The 2d chemical structure image of methanol is also called skeletal formula, which is the standard notation for organic molecules.
Structural formula and displayed formula for ethanol: Web 46k views 10 years ago. Mark charges on atoms if there are charges. Take a look at the following table for the same. In contrast, the structural formula (figure \(\pageindex{3b}\)) indicates how the atoms are connected, but it makes methanol look as if it is planar (which it is not). Condensed structural formulas are designed to be typed on a single line. Total number of electrons of the valance shells of ch 3 oh. It provides valuable insights into the molecule’s geometry, hybridization, and polarity. Condensed structural formula for ethanol: Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.
Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced active by default. Web the lewis structure of ch3oh, also known as methanol, is a representation of the molecule’s bonding and electron distribution. 141k views 10 years ago. Draw the molecule on the canvas by choosing buttons from the toc bond is active by default. Web i quickly take you through how to draw the lewis structure of ch3oh (methanol or methyl alcohol). In the lewis structure of structure there are a total of 14 valence electrons. Web steps to draw the lewis structure of methanol (ch3oh) 1) calculation of total valence electrons in methanol. Doc @ @ ®, h: I also go over hybridization, shape and bond angles. It is most commonly created by reacting precursor gases such as co and co 2 with h 2 hydrogen gas.
Methanol Molecule
Take a look at the following table for the same. It is not a hydrocarbon since the hydroxyl group is chemically bonded to the carbon atom. Would you expect to find this segment at the center or at the surface of a globular protein? Ch 3 oh — methyl alcohol: The carbon atoms in the chemical structure of methanol are.
FileMethanol structure.png Sciencemadness Wiki
It consists of a methyl group linked with a hydroxyl group. What are the rules for writing condensed structural formulas? This structure is also available as a 2d mol file or as a computed 3d sd file. Structural formula and displayed formula for ethanol: Web organic chemistry ways to draw and represent molecules condensed structure.
Structural chemical formula and model methanol Vector Image
Web condensed structural formula class of alcohol common name iupac name; Ch 3 ch 2 oh: Condensed structural formulas are designed to be typed on a single line. Web part a draw the condensed structural formulas of methanol and ethanol. Web the lewis structure of ch3oh, also known as methanol, is a representation of the molecule’s bonding and electron distribution.
Methanol Lewis Structure How to Draw the Lewis Structure for Methanol
In contrast, the structural formula (figure \(\pageindex{3b}\)) indicates how the atoms are connected, but it makes methanol look as if it is planar (which it is not). Web structural formula and displayed formula for methanol: Total number of electrons of the valance shells of ch 3 oh. Writing methanol as ch 4 o tells nothing about its structure. Web for.
Methanol Molecule, Scientific Molecular Model, 3d Rendering Stock Photo
Mark lone pairs on atoms. Web the formula for methanol is ch 3 oh. Web organic chemistry ways to draw and represent molecules condensed structure. Total number of electrons of the valance shells of ch 3 oh. Ch 3 ch 2 ch 2 ch 2 oh:
Methanol Formula Illustration Stock Illustration Illustration of line
It provides valuable insights into the molecule’s geometry, hybridization, and polarity. Web 46k views 10 years ago. It is not a hydrocarbon since the hydroxyl group is chemically bonded to the carbon atom. What are the rules for writing condensed structural formulas? Web organic chemistry ways to draw and represent molecules condensed structure.
Methanol Molecule Illustration Stock Illustration Illustration of
Web the formula for methanol is ch 3 oh. It provides valuable insights into the molecule’s geometry, hybridization, and polarity. Structural formula and displayed formula for ethanol: The 2d chemical structure image of methanol is also called skeletal formula, which is the standard notation for organic molecules. It is also known as wood alcohol or methyl alcohol.
FileMethanol flat structure.png Wikimedia Commons
It is also known as wood alcohol or methyl alcohol. Part a draw the expanded structural formula for methanol. Web the formula for methanol is ch 3 oh. Ch 3 ch 2 oh (molecular formula for ethanol c 2 h 6 o). Would you expect to find this segment at the center or at the surface of a globular protein?
Chemical Structural Formula Methanol Stock Vector (Royalty Free
Web the formula for methanol is ch 3 oh. Web 46k views 10 years ago. Web for example, the molecular formula for methanol (figure \(\pageindex{3a}\)) gives only the number of each kind of atom; It consists of a methyl group linked with a hydroxyl group. Ch 3 ch 2 ch 2 ch 2 oh:
Ch3oh methanol molecule Royalty Free Vector Image
It is most commonly created by reacting precursor gases such as co and co 2 with h 2 hydrogen gas. Mark lone pairs on atoms. The carbon atoms in the chemical structure of methanol are implied to be located at the corner (s) and hydrogen atoms attached to carbon atoms are not. Doc @ @ ®, h: Mark charges on.
Part A Draw The Expanded Structural Formula For Methanol.
Ch 3 ch 2 oh: Ch 3 ch 2 ch 2 oh: Web the lewis structure of ch3oh, also known as methanol, is a representation of the molecule’s bonding and electron distribution. Ch 3 oh — methyl alcohol:
It Provides Valuable Insights Into The Molecule’s Geometry, Hybridization, And Polarity.
Total number of electrons of the valance shells of ch 3 oh. The 3d structure may be viewed using java or. It consists of a methyl group linked with a hydroxyl group. Web structural formula and displayed formula for methanol:
Web For Example, The Molecular Formula For Methanol (Figure \(\Pageindex{3A}\)) Gives Only The Number Of Each Kind Of Atom;
Mark lone pairs on atoms. It is not a hydrocarbon since the hydroxyl group is chemically bonded to the carbon atom. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced active by default. 141k views 10 years ago.
Formal Charge On Oxygen =.
Condensed structural formula for ethanol: I also go over hybridization, shape and bond angles. In the lewis structure of structure there are a total of 14 valence electrons. Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.