Nitrogen Atom Drawing
Nitrogen Atom Drawing - The helium atom contains two protons and two electrons. Web drawing the lewis structure of nf3. Nitrogen gas (n 2) comprises 78.1% of the volume of the earth’s air. To draw the nitrogen bohr model, represent the 7 protons, 7 neutrons, and 7 electrons. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Web a neutral nitrogen atom has five valence electrons (it is in group 15). Schematic diagram of the hyperfine interaction between the reporter spin and the proton spins (the gold arrows). ) lewis structure | steps of drawing. Lewis structure of nh 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. The element atomic number and name are listed in the upper left.
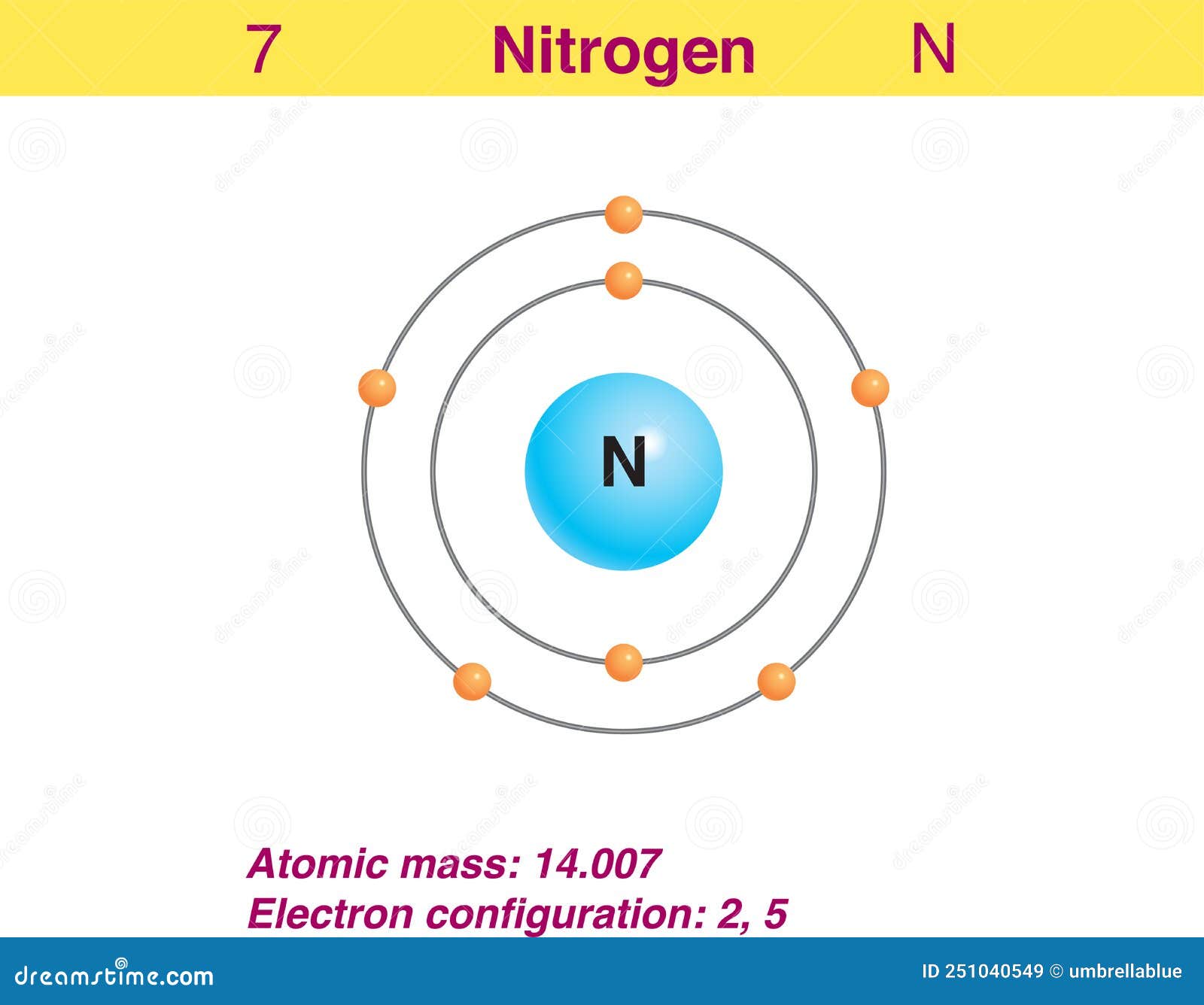
Web this diagram shows how the electrons in the nitrogen atom are arranged in different orbitals. The element atomic number and name are listed in the upper left. We’ll use a bohr diagram to visually represent where the electrons. Web single and multiple covalent bonds. The outermost shell in the bohr diagram of nitrogen contains 5 electrons that also called valence electrons. To create the lewis structure of nf3, follow these steps: A bohr diagram of the nitrogen atom. Web the electron configuration and the orbital diagram are: In the molecular orbital diagram of n2, the two nitrogen atoms are represented by their atomic orbitals. Web the nitrogen atom is depicted as the larger, central blue sphere, and the three hydrogen atoms are depicted as the smaller white spheres off to the sides, which form a kind of tripod.
Web the amine functional group is as follows: Begin by identifying the number of valence electrons for each atom in the nf3 molecule. The electron shells are shown, moving outward from the nucleus. I show you where nitrogen is on the periodic table and how to determine. The outermost shell in the bohr diagram of nitrogen contains 5 electrons that also called valence electrons. Schematic diagram of the hyperfine interaction between the reporter spin and the proton spins (the gold arrows). The element atomic number and name are listed in the upper left. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0, m s = + 1 2 m s = + 1 2). Nitrogen gas (n 2) comprises 78.1% of the volume of the earth’s air. The helium atom contains two protons and two electrons.
Electron of the Element Nitrogen Stock Vector Illustration of print
Nitrogen (n) is in group 15 of the periodic table and has 5 valence electrons, while fluorine (f) is in group 17 and possesses. It is used to make fertilisers, nitric acid, nylon, dyes and explosives. Lewis structure of nh 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. We’ll use a.
Chemical bonding Atomic structure and bonding Britannica
Amines are classified as primary, secondary, or tertiary by the number of hydrocarbon groups attached to the nitrogen atom. Two 2p orbitals and one 2s orbital. Nitrogen gas (n 2) comprises 78.1% of the volume of the earth’s air. Web drawing a molecular orbital diagram of n2 can provide valuable insights into its electronic structure and properties. ) lewis structure.
Nitrogen atom garetutah
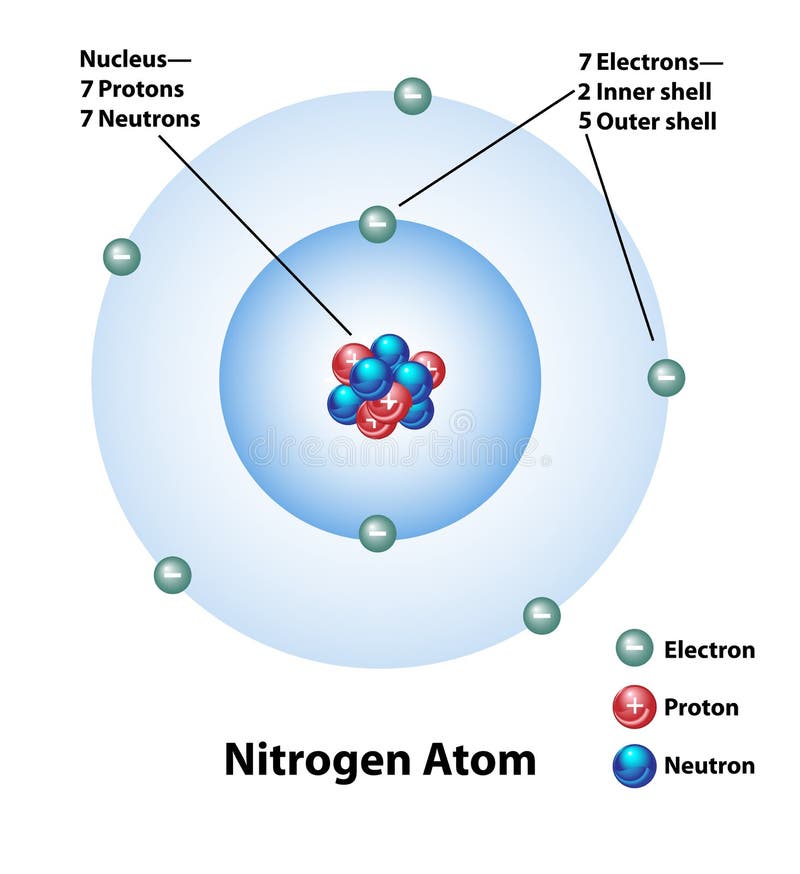
Web the amine functional group is as follows: The molecule is suitable for education or science and is isolated on a white background. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). It only appears in 0.002% of the earth's crust by mass. The final ring or shell of electrons contains the typical number of valence.
Diagram representation of the element nitrogen Vector Image
We’ll use a bohr diagram to visually represent where the electrons. Chemistry model molecule nitrogen n2. The helium atom contains two protons and two electrons. The cosmic abundance—the estimated total abundance in the universe—is between three and seven atoms per atom of silicon. This study highlights the role of halogen bond donors as catalytic promoters, aiding activation of n2.
Nitrogen atomic model
Naming and drawing amines is shared under a not declared license. Web single and multiple covalent bonds. Web in this video we'll look at the atomic structure and bohr model for the nitrogen atom (n). Begin by sketching the nucleus, and then draw the two electron shells. A bohr diagram of the nitrogen atom.
Nitrogen, atomic structure Stock Image C018/3688 Science Photo
The electrons present in the outermost shell of an atom. We’ll use a bohr diagram to visually represent where the electrons. Web the nv center comprises a nitrogen atom (the purple ball labeled n) and an adjacent lattice vacancy (gray ball labeled v). The principal axis lies along the connection line between nitrogen and the vacancy. The outermost shell in.
Nitrogen Facts, Symbol, Discovery, Properties, Uses
The outermost shell in the bohr diagram of nitrogen contains 5 electrons that also called valence electrons. Begin by identifying the number of valence electrons for each atom in the nf3 molecule. The electron shells are shown, moving outward from the nucleus. Orbital is the region of space around the nucleus of an atom where electrons are found. I show.
Nitrogen, atom model. Chemical element with symbol N and with atomic
From its lewis electron structure, the nitrogen atom in ammonia has one lone pair and shares three bonding pairs with hydrogen atoms, so nitrogen itself is assigned a total of five electrons [2 nonbonding e − + (6 bonding e − /2)]. The electron shells are shown, moving outward from the nucleus. This study highlights the role of halogen bond.
Bohr atomic model of a nitrogen atom. vector illustration for science
The electrons present in the outermost shell of an atom. Web nitrogen, nonmetallic element of group 15 [va] of the periodic table. The cosmic abundance—the estimated total abundance in the universe—is between three and seven atoms per atom of silicon. For any atom, stability is achieved by following the octet rule, which is to say all atoms (with a few.
Nitrogen Atom Diagram Stock Illustrations 119 Nitrogen Atom Diagram
Web a neutral nitrogen atom has five valence electrons (it is in group 15). Chemistry model molecule nitrogen n2. It is a colorless, odorless, tasteless gas that is the most plentiful element in earth’s atmosphere and is a constituent of all living matter. The element atomic number and name are listed in the upper left. Each step of drawing the.
The Electron Shells Are Shown, Moving Outward From The Nucleus.
Web download 120 nitrogen atom diagram stock illustrations, vectors & clipart for free or amazingly low rates! Web nitrogen atom doping is widely employed to prepare efficient adsorbents to handle target pollutants thanks to its similarity in radius to carbon atoms and excellent functionality. To write an orbital diagram of nitrogen, you first need to know the atomic orbitals and the orbital notation for the nitrogen atom, and also you need to know hund. Nitrogen is important to the chemical industry.
It Is A Colorless, Odorless, Tasteless Gas That Is The Most Plentiful Element In Earth’s Atmosphere And Is A Constituent Of All Living Matter.
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Web bacteria play a key role in the nitrogen cycle. Web nitrogen atom bohr model representation of the nitrogen atom, number 7 and symbol n. The outermost shell in the bohr diagram of nitrogen contains 5 electrons that also called valence electrons.
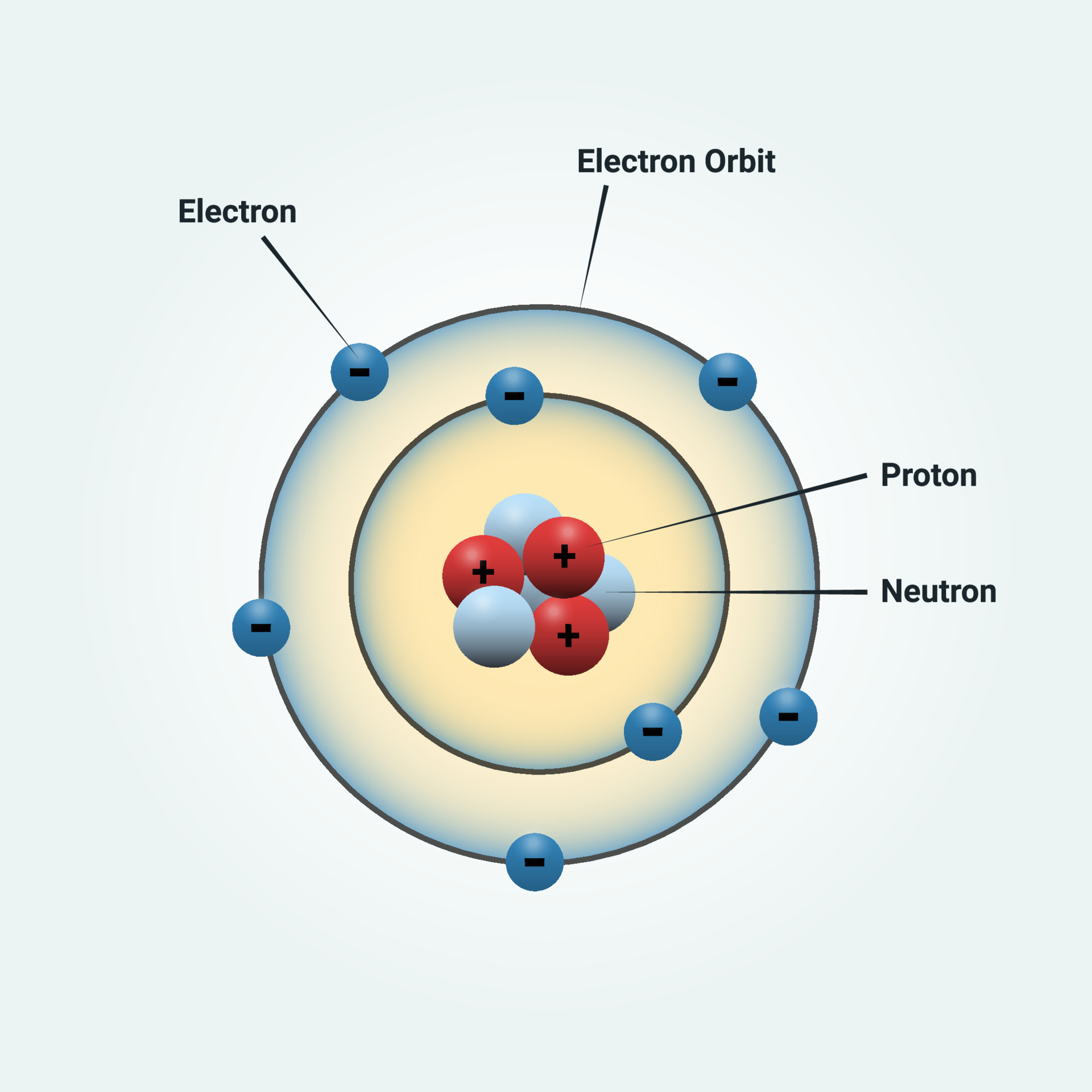
Surrounding This Nucleus Are Two Electron Shells, Containing A Total Of 7 Electrons.
Web the nitrogen atom is depicted as the larger, central blue sphere, and the three hydrogen atoms are depicted as the smaller white spheres off to the sides, which form a kind of tripod. Web single and multiple covalent bonds. Web the amine functional group is as follows: Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
Web Drawing A Molecular Orbital Diagram Of N2 Can Provide Valuable Insights Into Its Electronic Structure And Properties.
The cosmic abundance—the estimated total abundance in the universe—is between three and seven atoms per atom of silicon. 7), the most common isotope of the element nitrogen. The element atomic number and name are listed in the upper left. In the diagram above, we.