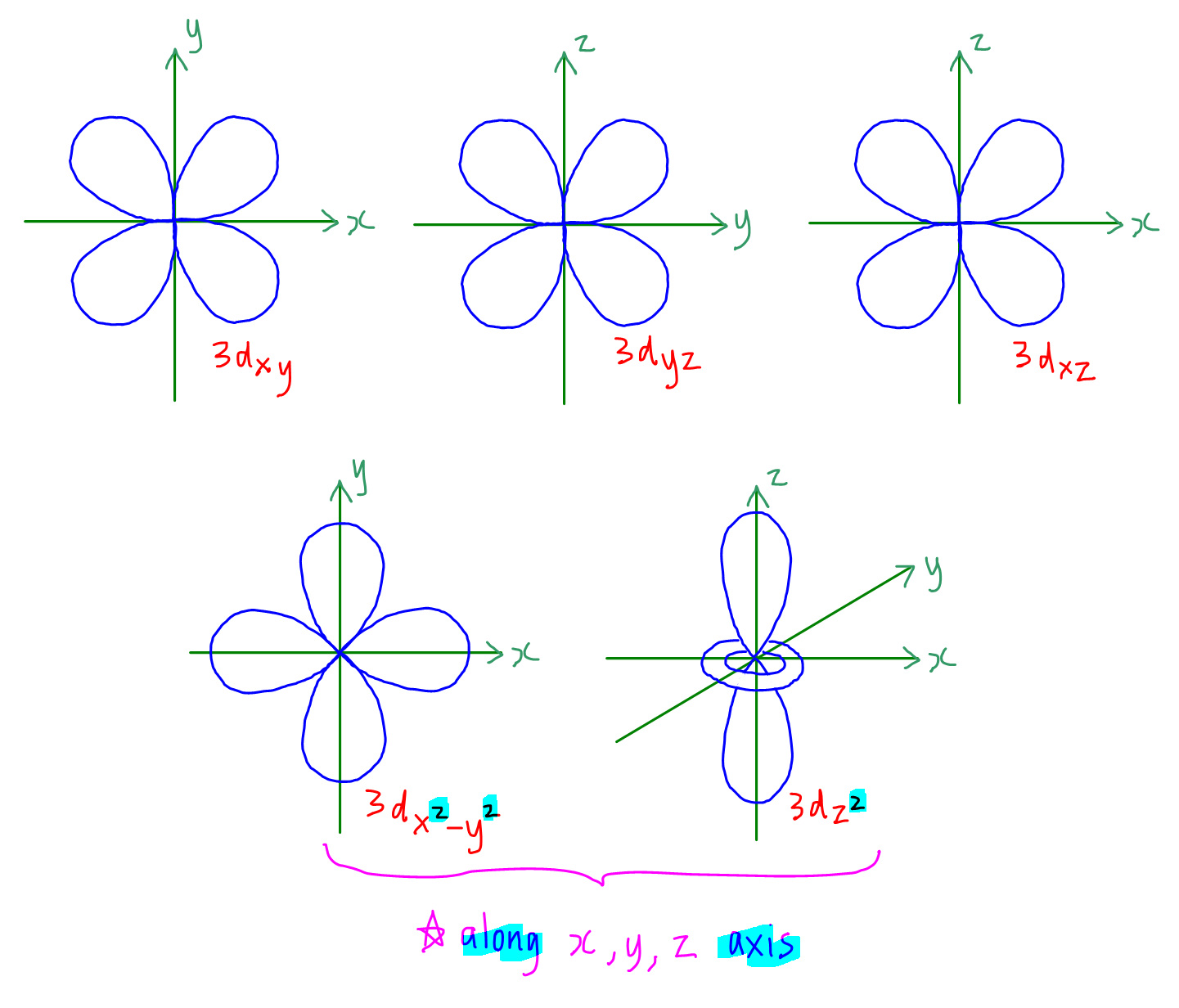
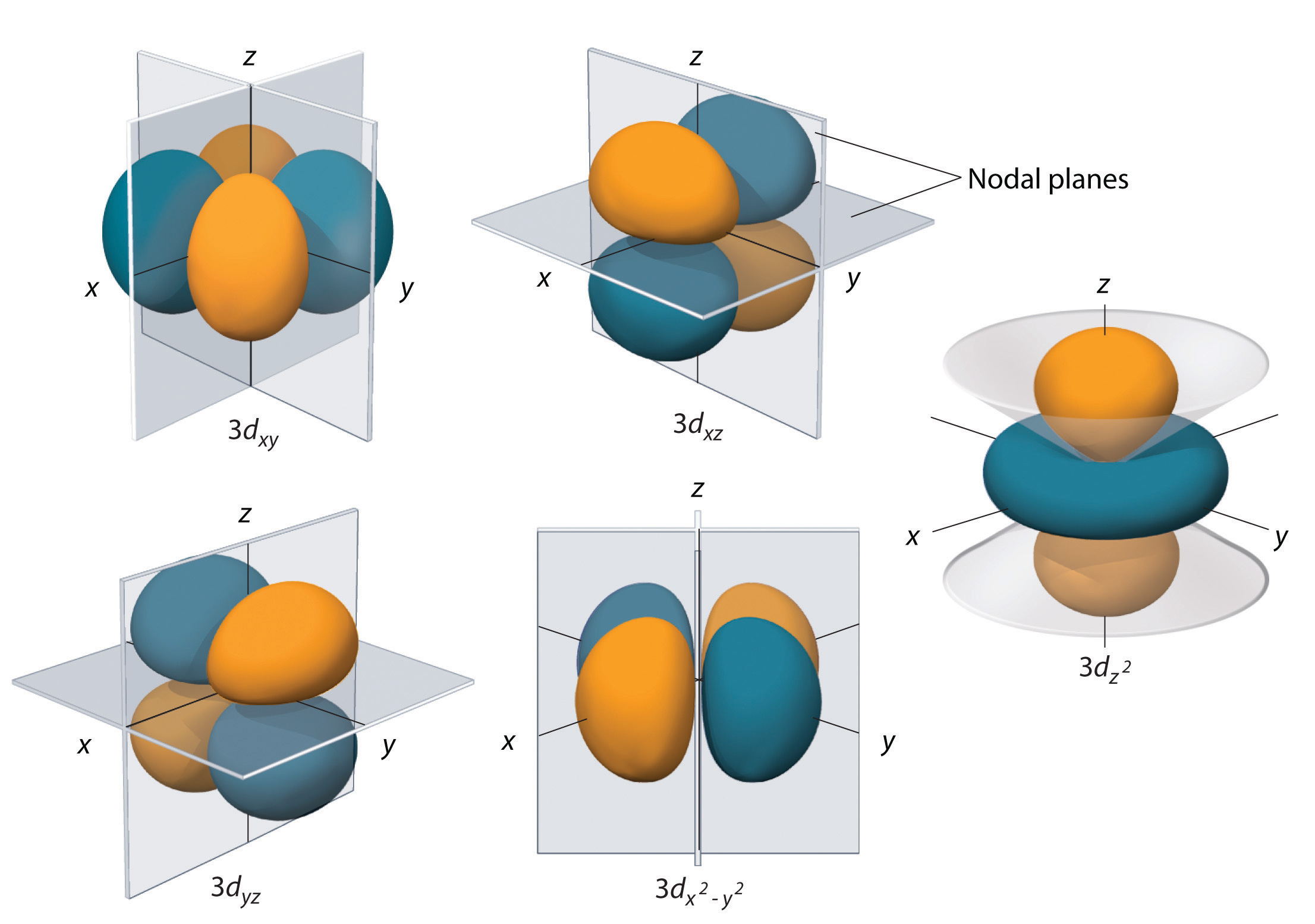
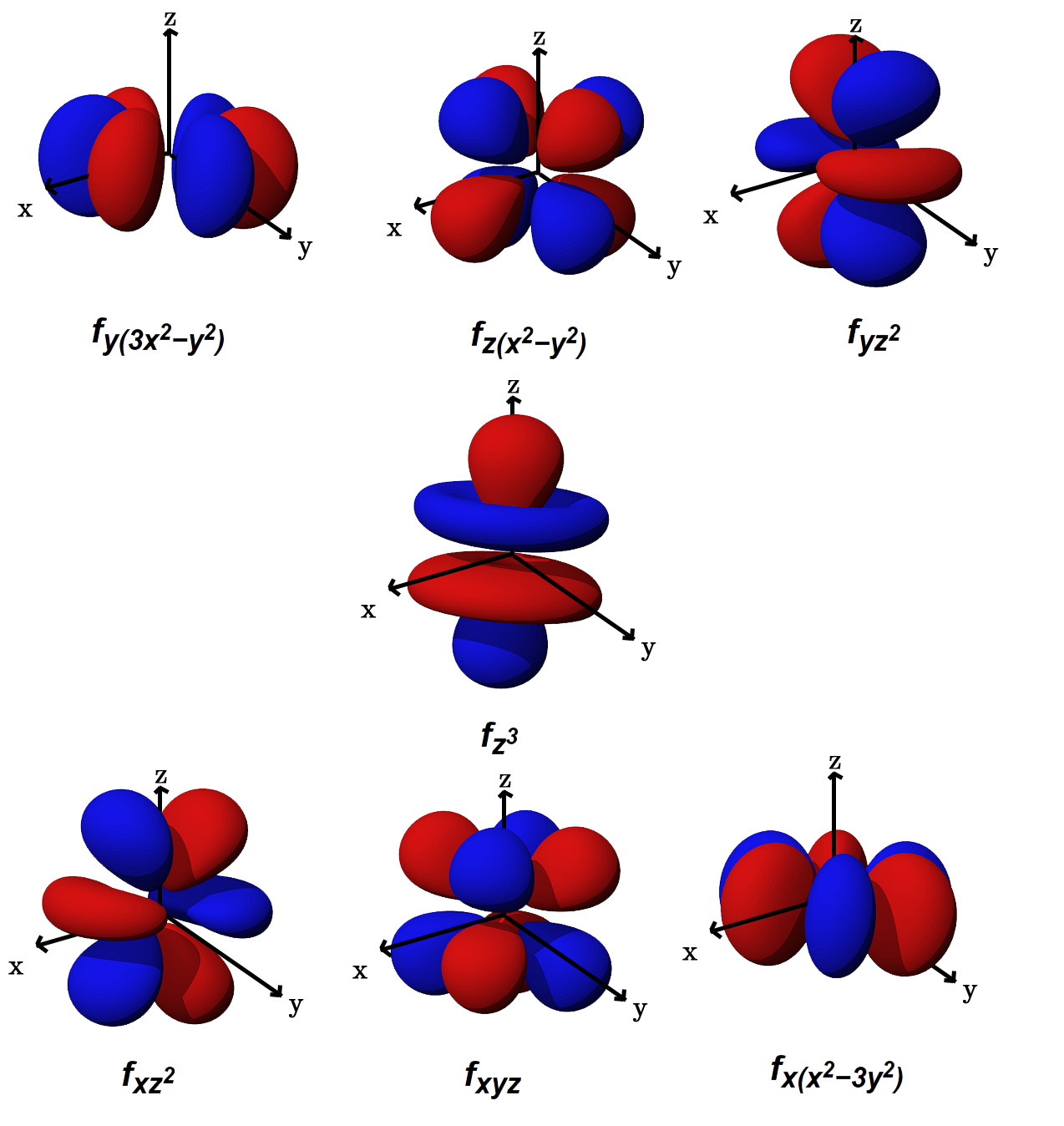
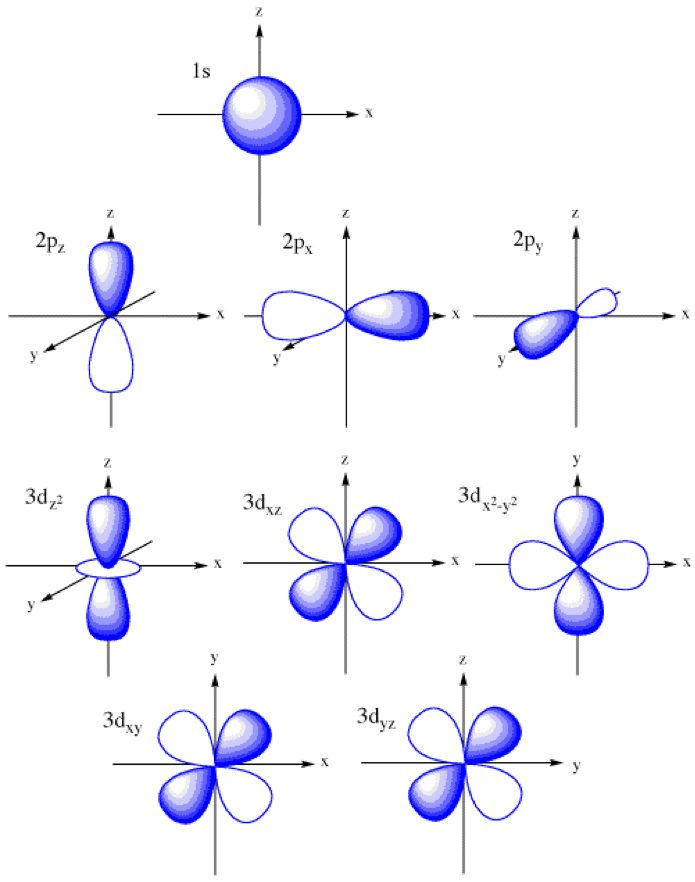
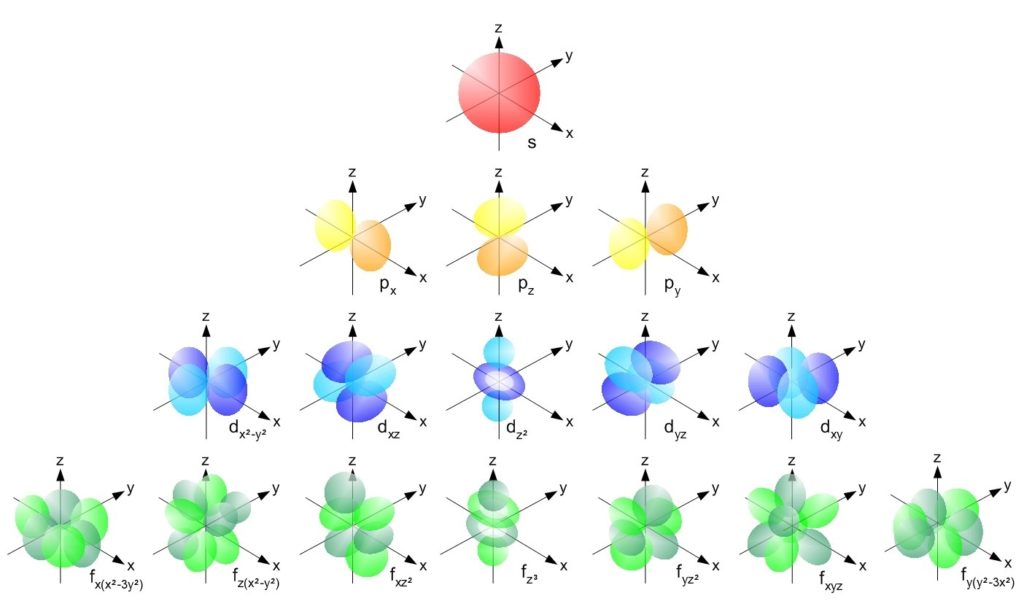
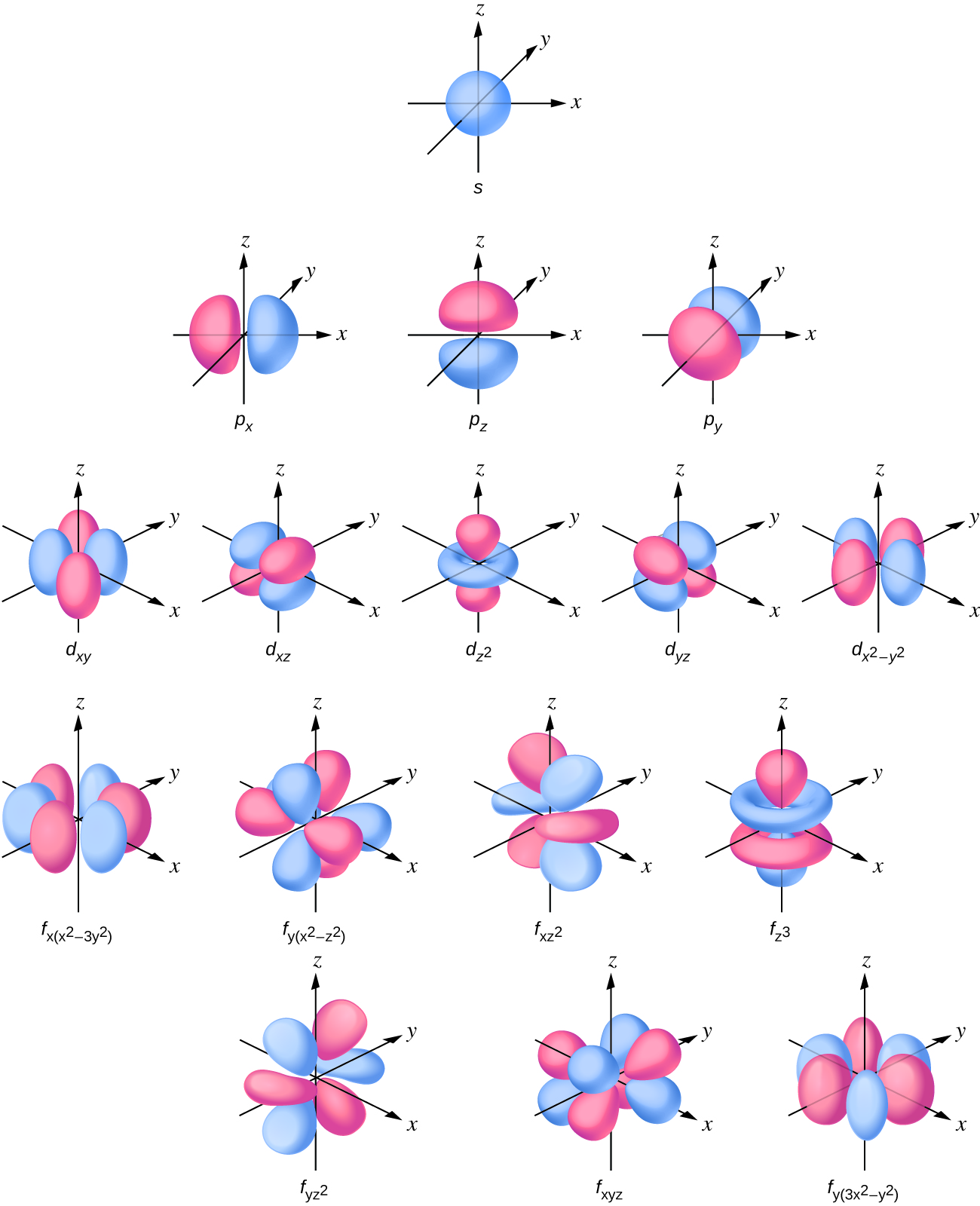
Orbital Drawings
Orbital Drawings - Web molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. Typically, they only show the outermost electrons. Web so when we say 1s or 3d xz we are orbital in terms of its location in space, and the images in figure \(\pageindex{1}\) represents the shapes of some common orbitals where there is roughly a 90% probability of finding the electron that resides in that orbital. Every unique orbital can only contain up to two electrons. Web an orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A single orbital cannot contain more than 2 electrons of opposite spin. The word orbital is used in order to make a distinction between these wave patterns and the circular or elliptical orbits of the bohr picture shown in the wave nature of the electron. Web the orbital diagram (also called an energy diagram) is another way of writing the electronic configuration of an element, but representing the electrons with small arrows and the orbitals with small horizontal lines or boxes. Web an orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals.
A box, line, or circle, is drawn to represent each orbital in the electron configuration. Web molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explores s and p orbitals in some detail, including their shapes and energies. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space with different probabilities of containing an electron. Get a pencil (preferably a mechanical pencil for precision), a good eraser, a ruler, and a sheet of drawing paper. In an orbital diagram, an electron is represented by an arrow, while a box represents an atomic orbital. A single orbital cannot contain more than 2 electrons of opposite spin. Orbital diagrams must follow 3 rules: Discover the three rules for creating electron orbital charts, and study examples of filling electron orbital in a diagram.
Web this page discusses atomic orbitals at an introductory level. Web learn how to draw orbital diagrams. A single orbital cannot contain more than 2 electrons of opposite spin. Web an orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. General chemistry start typing, then use the up and down arrows to select an option from the list.? Web an orbital is the quantum mechanical refinement of bohr’s orbit. Orbital diagrams must follow 3 rules: It explores s and p orbitals in some detail, including their shapes and energies. Discover the three rules for creating electron orbital charts, and study examples of filling electron orbital in a diagram. Web how to draw orbital diagrams.
Biochemistry Glossary Orbitals 2. Shape Draw It to Know It
Web how to draw orbital diagrams. Discover the three rules for creating electron orbital charts, and study examples of filling electron orbital in a diagram. A single orbital cannot contain more than 2 electrons of opposite spin. Web molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. In orbitals diagrams, the orbitals are shown.
How to Draw Shapes of Orbitals
D orbitals are described only in terms of their energy, and f orbitals are only mentioned in passing. The site includes opportunities to practice filling in electrons, attaching the names/symbols of mos, and matching orbital overlap drawings to mos. Web how to draw orbital diagrams. Orbital diagrams provide a visual representation of how electrons are distributed within an atom. In.
CH 878 SHAPES OF ATOMIC ORBITALS Dbios Charts
Web the orbital diagram or orbital notation simply represents the arrangement of electrons in different orbitals of an atom. D orbitals are described only in terms of their energy, and f orbitals are only mentioned in passing. Web the orbital diagram (also called an energy diagram) is another way of writing the electronic configuration of an element, but representing the.
Shapes of Atomic Orbitals — Overview & Examples Expii
Web so when we say 1s or 3d xz we are orbital in terms of its location in space, and the images in figure \(\pageindex{1}\) represents the shapes of some common orbitals where there is roughly a 90% probability of finding the electron that resides in that orbital. Each box represents one orbital, and each arrow indicates one electron. General.
Radial and Angular Parts of Atomic Orbitals Chemistry LibreTexts
This article will explore the basics of how to draw each type of diagram, and important rules to follow in their construction. Typically, they only show the outermost electrons. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. (using the aufau principle to order the orbitals and hence the.
Shapes of Atomic Orbitals — Overview & Examples Expii
In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space with different probabilities of containing an electron. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Web how to draw orbital diagrams. It explains how to write the.
Atom Orbitals Chart
Web an orbital is the quantum mechanical refinement of bohr’s orbit. This will represent the nucleus of the atom. (using the aufau principle to order the orbitals and hence the boxes, lines or circles, as shown below) Web an orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within.
Atomic orbitals explained polizhuge
Every unique orbital can only contain up to two electrons. There are three different rules used for constructing an atomic orbital diagram. Web how to draw orbital diagrams. This is a way of showing the electron configuration of the atom. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration.
Atomic Orbital Types, Energies, Diagram, Examples, Summary
The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. A box, line, or circle, is drawn to represent each orbital in the electron configuration. Give them a try here: Web how to draw orbital diagrams. In contrast to his concept of a simple circular orbit with a.
6.6 The Shapes of Atomic Orbitals Chemistry LibreTexts
Discover the three rules for creating electron orbital charts, and study examples of filling electron orbital in a diagram. Web the orbital diagram (also called an energy diagram) is another way of writing the electronic configuration of an element, but representing the electrons with small arrows and the orbitals with small horizontal lines or boxes. A single orbital cannot contain.
It Explains How To Write The Orbital Diagram N.
Web this page discusses atomic orbitals at an introductory level. Web molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. They are typically drawn as 3d space around the nucleus and.
Electron Shells Consist Of One Or More Subshells, And Subshells Consist Of One Or More Atomic Orbitals.
The aufbau principle, the pauli exclusion principle and hund's. The word orbital is used in order to make a distinction between these wave patterns and the circular or elliptical orbits of the bohr picture shown in the wave nature of the electron. Discover the three rules for creating electron orbital charts, and study examples of filling electron orbital in a diagram. Web drawing an orbital diagram for an element like zinc (zn) can be a useful tool in understanding its electron configuration.
Web Learn How To Draw Orbital Diagrams.
Web the orbital diagram or orbital notation simply represents the arrangement of electrons in different orbitals of an atom. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space with different probabilities of containing an electron. Orbital diagrams provide a visual representation of how electrons are distributed within an atom. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space with different probabilities of containing an electron.
Web Orbital Diagrams Are A Visual Way To Show Where The Electrons Are Located Within An Atom.
Typically, they only show the outermost electrons. (using the aufau principle to order the orbitals and hence the boxes, lines or circles, as shown below) This is a way of showing the electron configuration of the atom. Web an orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals.