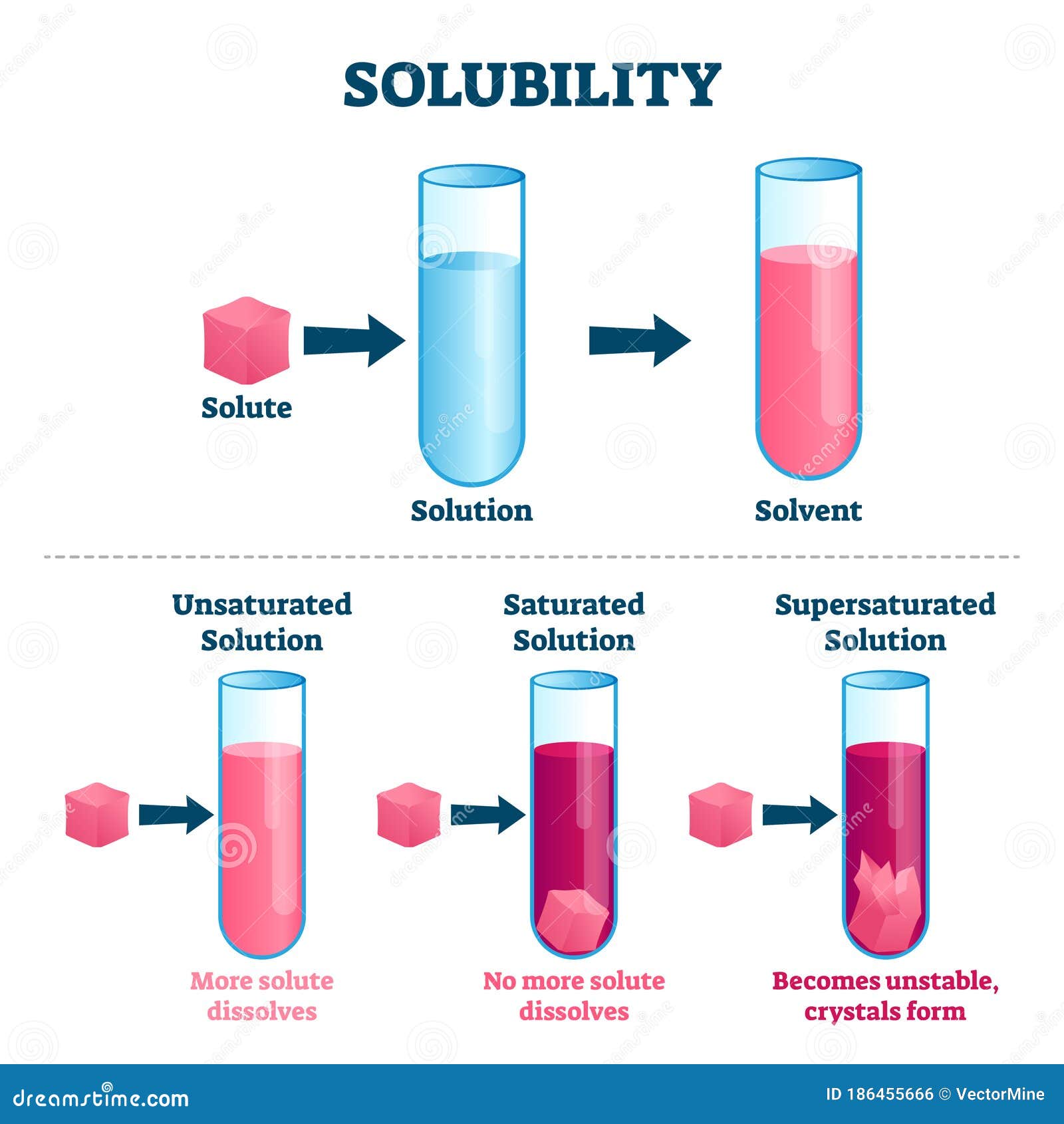
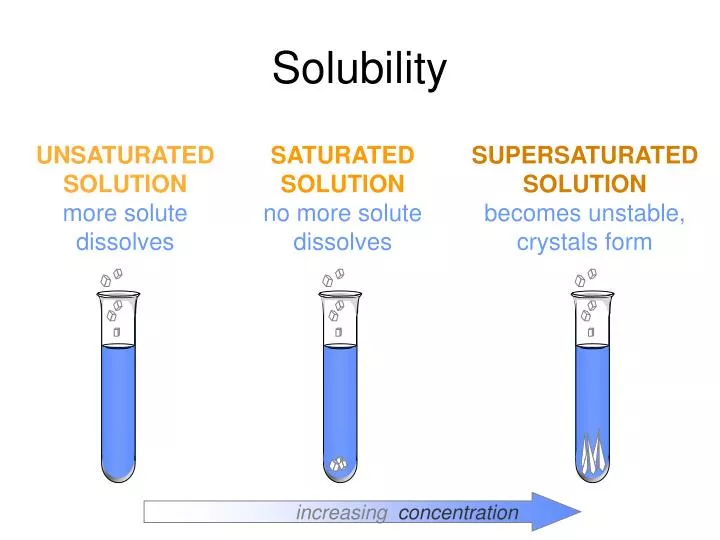
Solubility Drawing
Solubility Drawing - Properly use an analytical balance to measure mass. Web it is obvious that for a given density, the solubility increases due to rising temperature or, at constant temperature, almost linear with the solvent's density and hence solvent power. Send feedback | visit wolfram|alpha. Get the free solubility widget for your website, blog, wordpress, blogger, or igoogle. Want to join the conversation? This chemistry video tutorial provides a basic introduction into solubility curves. In such an equilibrium, le chatelier's principle can be used to explain most of the main factors that affect solubility. Web the definition of a solubility curve, sometimes also referred to as a solubility graph, is the relationship between solute and solvent at certain temperatures. This is not, however, a unidirectional process. Generate a workbook using google sheets.
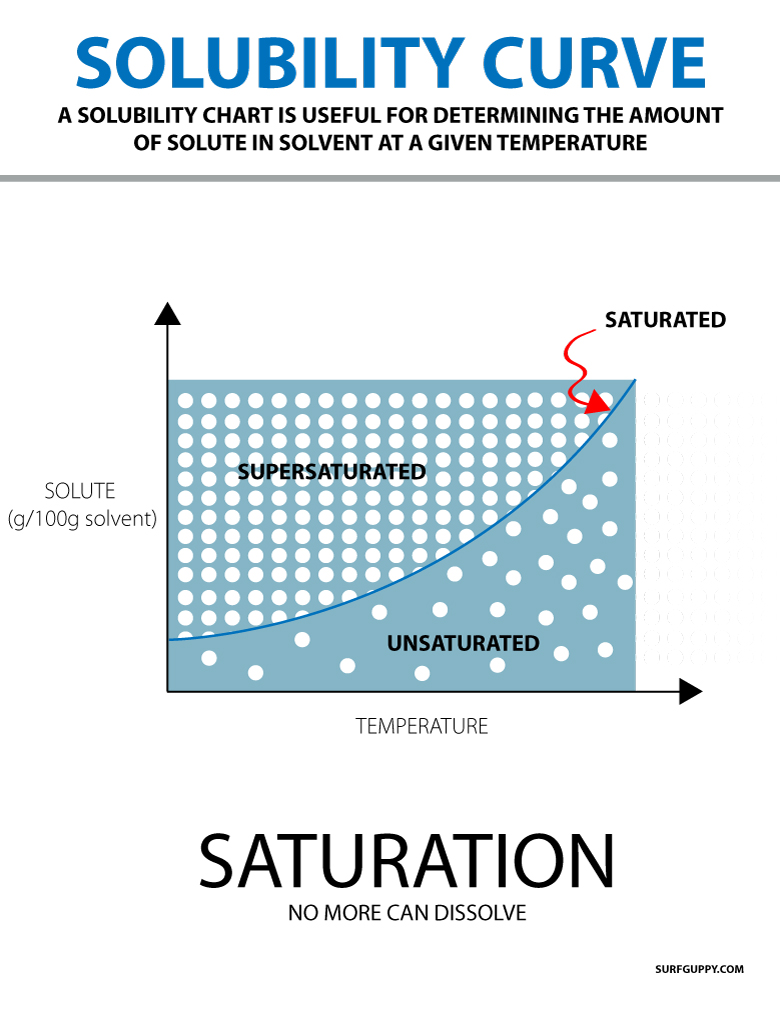
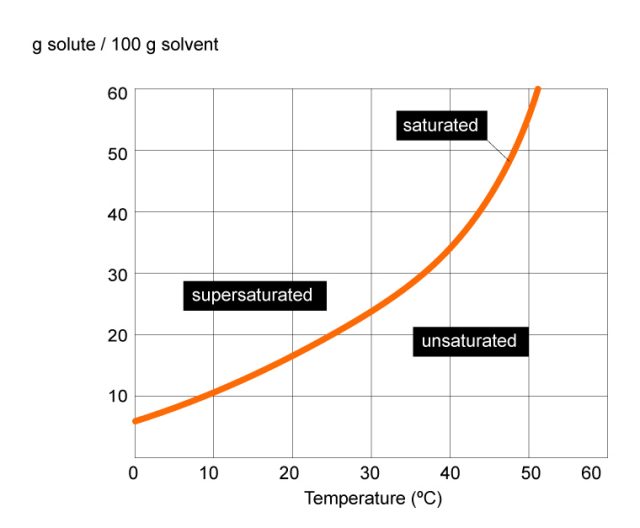
197k views 6 years ago new ap & general chemistry video playlist. Web the definition of a solubility curve, sometimes also referred to as a solubility graph, is the relationship between solute and solvent at certain temperatures. The five major types of chemical reactions are synthesis, decomposition, single replacement, double replacement, and combustion. Web solubility is often expressed as the mass of solute per volume (g/l) or mass of solute per mass of solvent (g/g), or as the moles of solute per volume (mol/l). Set up an experimental work station to measure the solubility of a salt in water as a function of the temperature. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Web to save your graphs! Web solubility graphs represent the relationship between solubility (in grams of solid per volume of water) vs temperature. Substances with similar polarities tend to be soluble in one another (like dissolves like). First, the epa is finalizing the repeal of the affordable clean energy (ace).
The resulting substance is called a solution. This chemistry video tutorial provides a basic introduction into solubility curves. First, the epa is finalizing the repeal of the affordable clean energy (ace). Want to join the conversation? Generally, the solute is a solid and the solvent is a liquid, such as our salt in water example above. Web it is obvious that for a given density, the solubility increases due to rising temperature or, at constant temperature, almost linear with the solvent's density and hence solvent power. Properly use an analytical balance to measure mass. The five major types of chemical reactions are synthesis, decomposition, single replacement, double replacement, and combustion. Web apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. Make sure you thoroughly understand the following essential ideas:
Solubilidad Dibujo
Calculate the amount of excess solute that remains undissolved in the resultant solution. Send feedback | visit wolfram|alpha. Make sure you thoroughly understand the following essential ideas: Nonpolar substances are generally more soluble in nonpolar solvents, while polar and ionic substances are generally more soluble in polar solvents. It is a graph where the.
Solubility and Precipitation Chemistry LibreTexts
Nonpolar substances are generally more soluble in nonpolar solvents, while polar and ionic substances are generally more soluble in polar solvents. Even for very soluble substances, however, there is usually a limit to how much solute can dissolve in a given quantity of solvent. Web the curve line drawn on a graph showing the relationship between temperature and solubility of.
Solubility Surfguppy Chemistry made easy for visual learners
Calculate the amount of excess solute that remains undissolved in the resultant solution. Substances with similar polarities tend to be soluble in one another (like dissolves like). Web collect experimental data and create a solubility curve. Want to join the conversation? Make sure you thoroughly understand the following essential ideas:
Chemistry Solutions And Mixtures Level 2 activity for kids
Web solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. Web chem1 (lower) 12: Web solubility is often expressed as the mass of solute per volume (g/l) or mass of solute per mass of solvent (g/g), or as the moles of solute per volume (mol/l). Find more chemistry.
Solubility Surfguppy Chemistry made easy visual learning
Send feedback | visit wolfram|alpha. Web calculate solubility for most materials. This chemistry video tutorial provides a basic introduction into solubility curves. Web solubility is a substance's ability to be dissolved. For example, both table salt (nacl) and table sugar (c 11 h 22 o 11 ) are soluble substances in water.
Solubility Surfguppy Chemistry made easy for visual learners
This chemistry video tutorial provides a basic introduction into solubility curves. 197k views 6 years ago new ap & general chemistry video playlist. Want to join the conversation? Make sure you thoroughly understand the following essential ideas: Web solubility graphs represent the relationship between solubility (in grams of solid per volume of water) vs temperature.
Solubility Vector Illustration. Labeled Solute, Solvent and Solution
Web solubility graphs represent the relationship between solubility (in grams of solid per volume of water) vs temperature. Web to save your graphs! The five major types of chemical reactions are synthesis, decomposition, single replacement, double replacement, and combustion. Web collect experimental data and create a solubility curve. This is not, however, a unidirectional process.
PPT Solubility PowerPoint Presentation, free download ID1115118
Web it is obvious that for a given density, the solubility increases due to rising temperature or, at constant temperature, almost linear with the solvent's density and hence solvent power. Web solubility rules a solute is considered soluble if an appreciable amount of it can be dissolved in a given amount of the solvent. Web the definition of a solubility.
Solution, Solubility, and Solubility Equilibrium Concept Organic
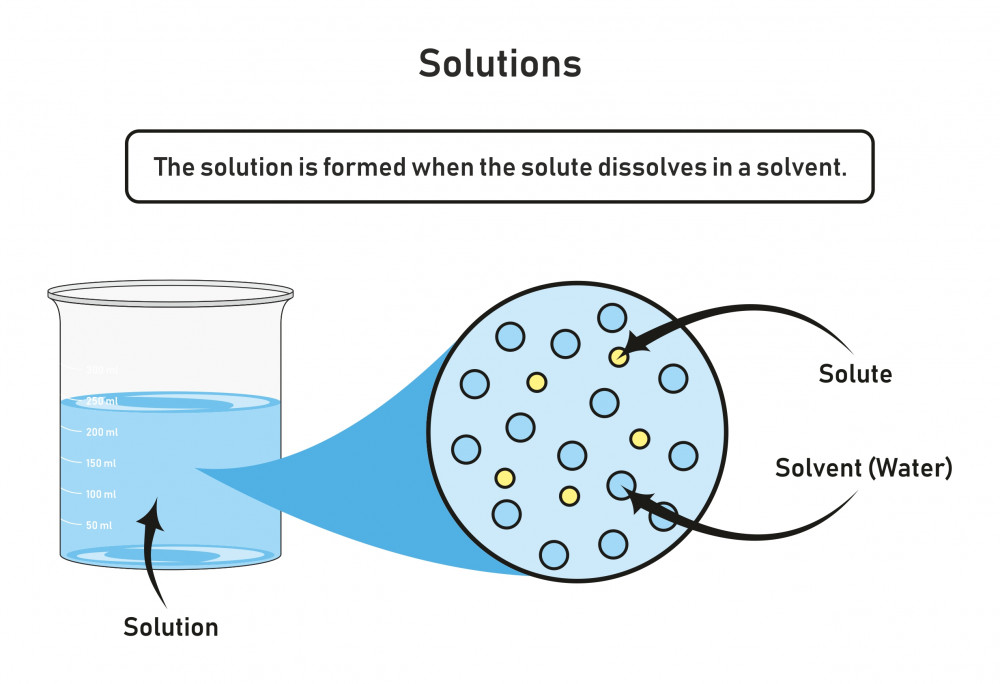
For example, both table salt (nacl) and table sugar (c 11 h 22 o 11 ) are soluble substances in water. When a solute dissolves, its individual atoms, molecules, or ions interact with the solvent, become solvated, and are able to diffuse independently throughout the solution (part (a) in figure 13.4). Web to save your graphs! To understand the relationship.
Solubility Infographic Infogram
Generate a workbook using google sheets. Web the definition of a solubility curve, sometimes also referred to as a solubility graph, is the relationship between solute and solvent at certain temperatures. Make sure you thoroughly understand the following essential ideas: The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in.
Send Feedback | Visit Wolfram|Alpha.
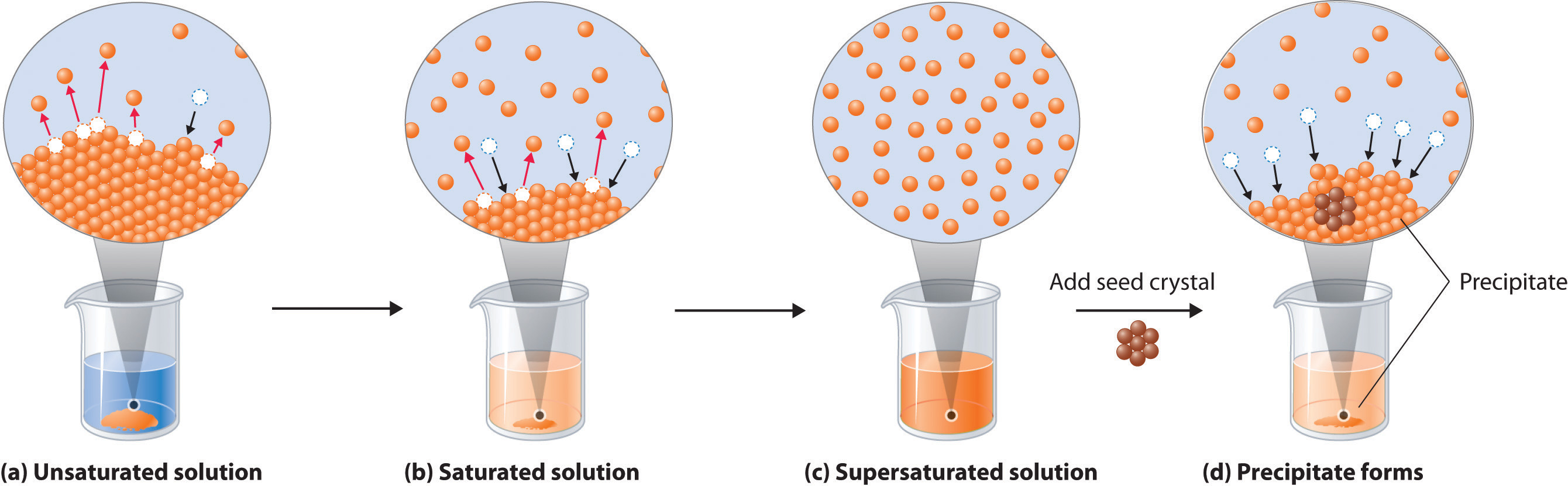
The substance that is dissolved is called a solute, and the substance it is dissolving in is called a solvent. In such an equilibrium, le chatelier's principle can be used to explain most of the main factors that affect solubility. First, the epa is finalizing the repeal of the affordable clean energy (ace). It is a graph where the.
Generally, The Solute Is A Solid And The Solvent Is A Liquid, Such As Our Salt In Water Example Above.
Draw a line to indicate the solubility of co 2 (g) versus temperature on the axes drawn in part a. Explore math with our beautiful, free online graphing calculator. If the solution is above the solubility line it is supersaturate and below the solubility line it is unsaturated. Substances with similar polarities tend to be soluble in one another (like dissolves like).
Want To Join The Conversation?
Web calculate solubility for most materials. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. Web apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. The resulting substance is called a solution.
Web Solubility Rules A Solute Is Considered Soluble If An Appreciable Amount Of It Can Be Dissolved In A Given Amount Of The Solvent.
Get the free solubility widget for your website, blog, wordpress, blogger, or igoogle. Web solubility is often expressed as the mass of solute per volume (g/l) or mass of solute per mass of solvent (g/g), or as the moles of solute per volume (mol/l). Web the curve line drawn on a graph showing the relationship between temperature and solubility of the substance at different temperatures is called a solubility curve. Web it is obvious that for a given density, the solubility increases due to rising temperature or, at constant temperature, almost linear with the solvent's density and hence solvent power.