Which Element Reacts With Oxygen To Form Ionic Bonds
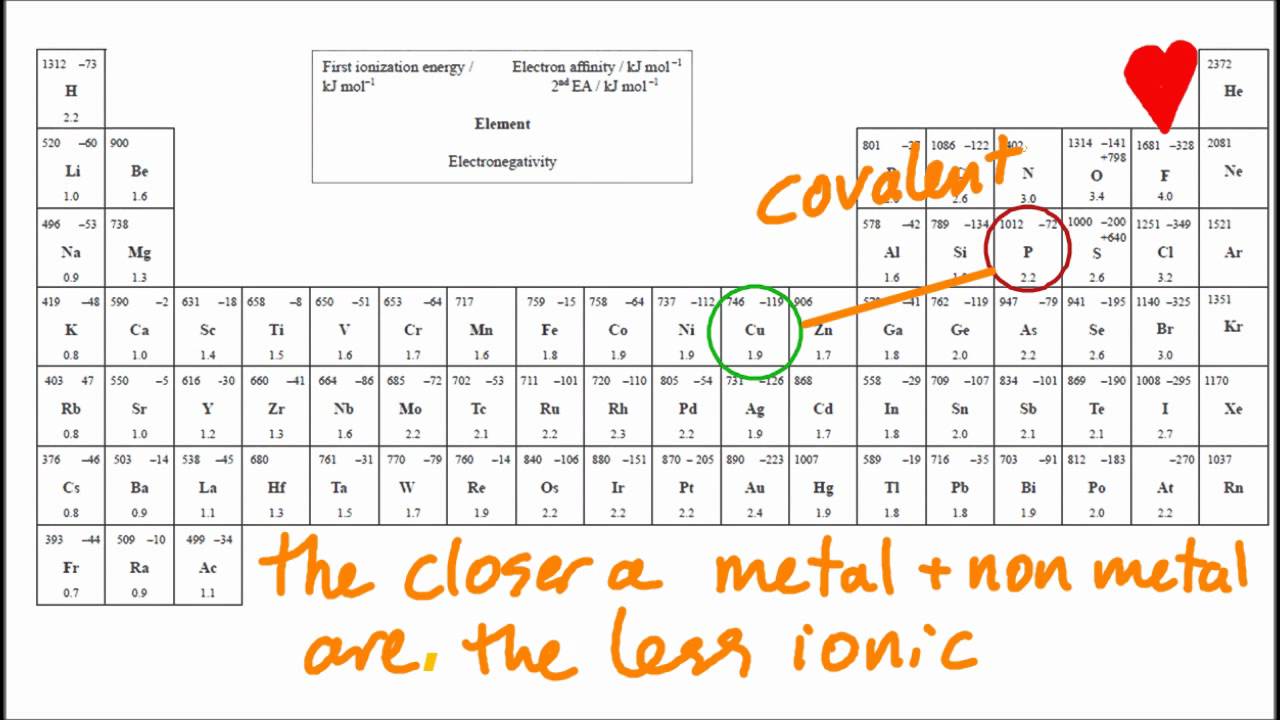
Which Element Reacts With Oxygen To Form Ionic Bonds - Web according to the electronic configuration, elements with 1 or 2 valence electrons react with oxygen to form ionic bonds.what is electronic configuration?electronic Web answered • expert verified. Define ionic and molecular (covalent) compounds predict the type of compound formed from elements. A lithium atom will lose 1 electron to form a stable 1 + ion. Web the positive/negative charge attraction would hold the two ions together. Lithium is in group 1 of the periodic table. A) calcium b)hydrogen c)chlorine d)nitrogen Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Web with metals, oxygen forms oxides that are largely ionic in character. The electronegativity of oxygen is 3.5 therefore any of the alkali or alkaline metals will ionically bond with oxygen to form an ionic compound.
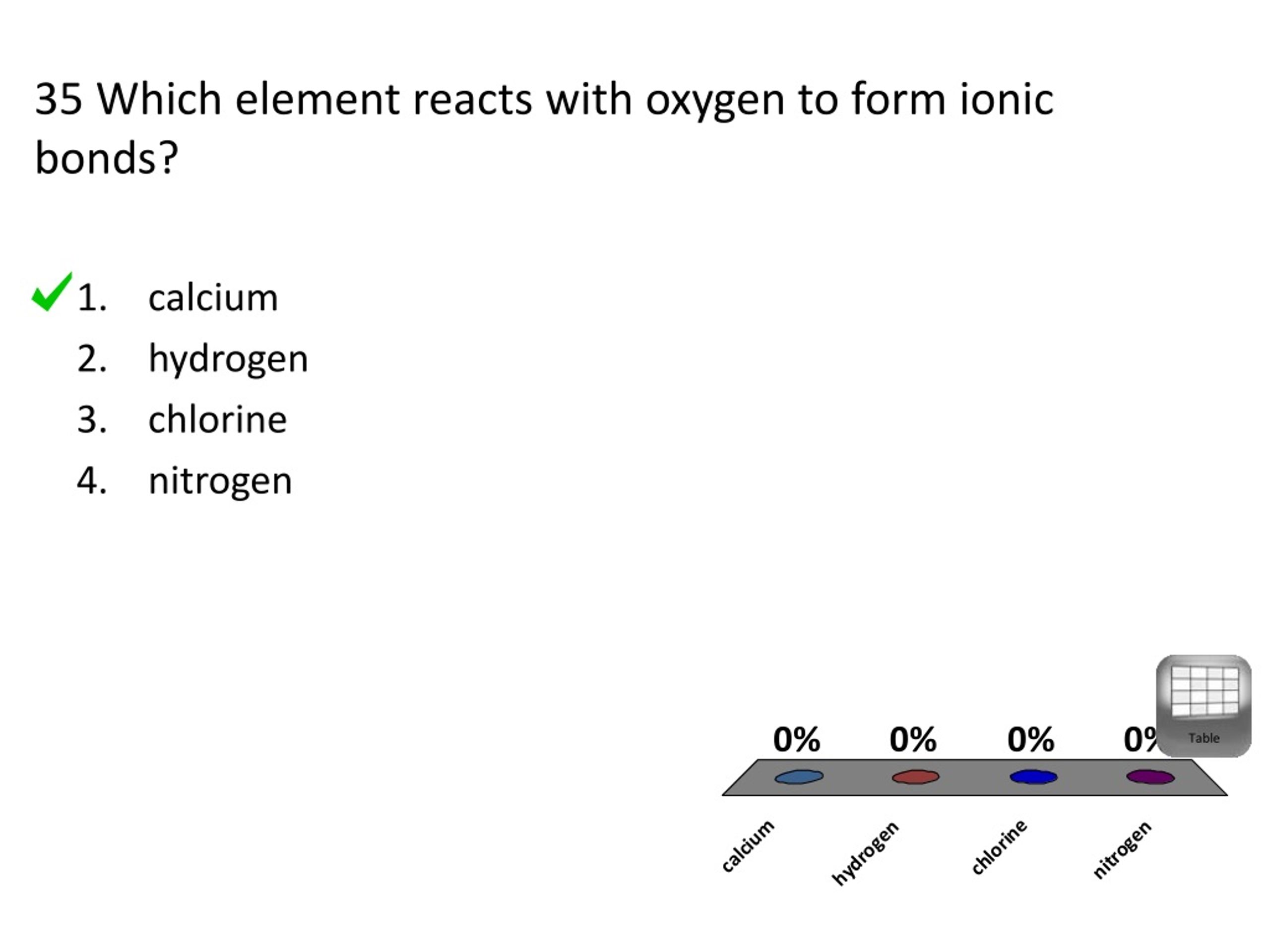
Web chemistry identify the element in period 3 of the periodic table that reacts with oxygen to form an ionic compound represented by the formula x2o. The electronegativity of oxygen is 3.5 therefore any of the alkali or alkaline metals will ionically bond with oxygen to form an ionic compound. Web which element reacts with oxygen to form ionic bonds? Web the positive/negative charge attraction would hold the two ions together. Even though no oxides of krypton are known, oxygen is able to form. Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond? An ionic compound is formed when there is a reaction between. Which element reacts with oxygen to form ionic bonds? Web which element reacts with oxygen to form ionic bonds? (1) calcium (3) chlorine (2) hydrogen (4) nitrogen.
(1) calcium (3) chlorine (2) hydrogen (4) nitrogen. Lithium is in group 1 of the periodic table. A metal and a nonmetal or polyatomic ions. (1) calcium (3) chlorine (2) hydrogen (4) nitrogen. Web answered • expert verified. Web chemistry identify the element in period 3 of the periodic table that reacts with oxygen to form an ionic compound represented by the formula x2o. A) calcium b)hydrogen c)chlorine d)nitrogen Web learning objectives by the end of this section, you will be able to: Which of the following is false concerning this interaction? Web which element reacts with oxygen to form ionic bonds?
Is O2 ionic or covalent? Techiescientist
Web calcium reacts with oxygen to form an ionic bond. Web oxygen can form oxides with heavier noble gases xenon and radon, although this needs indirect methods. Web according to the electronic configuration, elements with 1 or 2 valence electrons react with oxygen to form ionic bonds.what is electronic configuration?electronic There are general trends in the reactions between main group.
Solved Halogens can react with each other to form A)
Lithium is in group 1 of the periodic table. The two most basic types of. The magnesium would lose two electrons,. Web learning objectives by the end of this section, you will be able to: Which element reacts with oxygen to form ionic bonds?
Sodium Reacts With Oxygen / Ionic compound for sodium oxide Science
(1) calcium (3) chlorine (2) hydrogen (4) nitrogen. Web up to 6% cash back sodium will react with oxygen and form an ionic compound. Web which element reacts with oxygen to form ionic bonds? Oxygen is in group 6 of the. An ionic bond by definition is the kind of chemical bond formed when there is a complete transfer of.
PPT The University of the State of New York REGENTS HIGH SCHOOL
Web up to 6% cash back sodium will react with oxygen and form an ionic compound. A lithium atom will lose 1 electron to form a stable 1 + ion. Web the positive/negative charge attraction would hold the two ions together. Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond?.
Ionic Bond Definition, Types, Properties & Examples
The two most basic types of. An ionic bond by definition is the kind of chemical bond formed when there is a complete transfer of valence electrons from one. Web learning objectives by the end of this section, you will be able to: Web with metals, oxygen forms oxides that are largely ionic in character. Web oxygen can form oxides.
chemistry Can carbon and titanium form an ionic bond
Web calcium reacts with oxygen to form an ionic bond. Web which element reacts with oxygen to form ionic bonds? 35 which element reacts with oxygen to form ionic bonds? (1) calcium (3) chlorine (2) hydrogen (4) nitrogen. Which of the following is false concerning this interaction?
4.2 Predict if a compound of 2 elements is ionic using the table of EN
Web up to $3 cash back get the detailed answer: Web learning objectives by the end of this section, you will be able to: Web answered • expert verified. Even though no oxides of krypton are known, oxygen is able to form. Lithium is in group 1 of the periodic table.
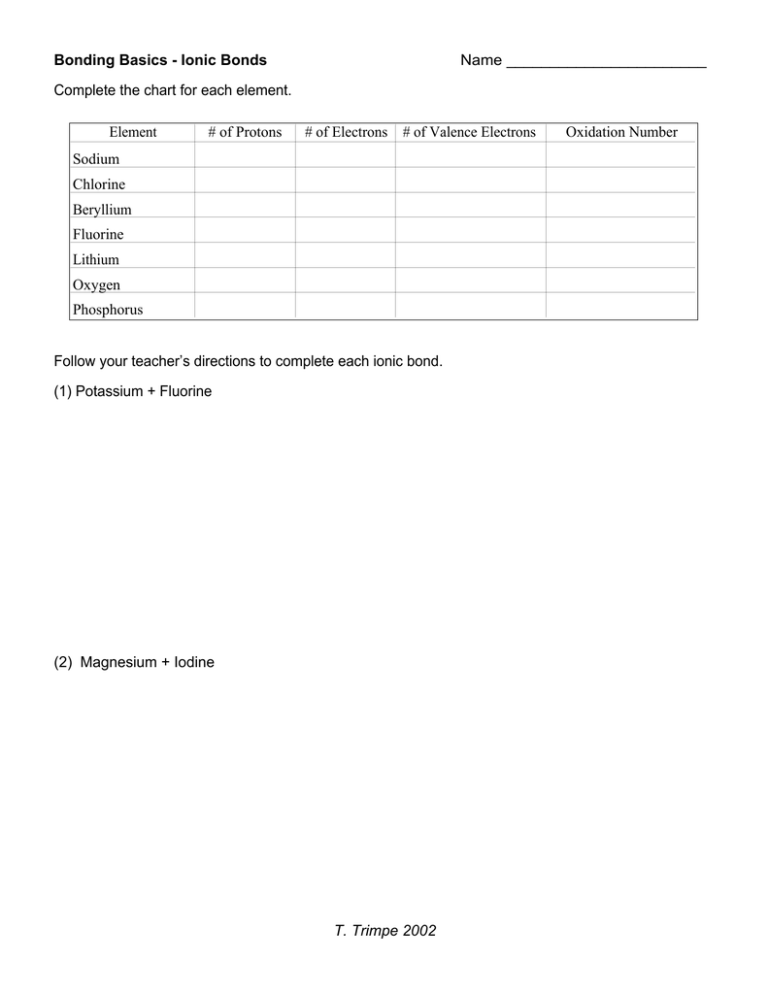
Bonding Basics Ionic Bonds Name _______________________ Element
A lithium atom will lose 1 electron to form a stable 1 + ion. Web answered • expert verified. Define ionic and molecular (covalent) compounds predict the type of compound formed from elements. Which element reacts with oxygen to form ionic bonds? Web with metals, oxygen forms oxides that are largely ionic in character.
Oxygen form Periodic Table of Elements — Stock Photo © fambros 6284480
Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond? The electronegativity of oxygen is 3.5 therefore any of the alkali or alkaline metals will ionically bond with oxygen to form an ionic compound. Which element reacts with oxygen to form ionic bonds? Web oxygen can form oxides with heavier noble.
Is SiO2 Ionic or Covalent? Techiescientist
Oxygen is in group 6 of the. Web calcium reacts with oxygen to form an ionic bond. An ionic bond by definition is the kind of chemical bond formed when there is a complete transfer of valence electrons from one. Web chemistry identify the element in period 3 of the periodic table that reacts with oxygen to form an ionic.
An Ionic Bond By Definition Is The Kind Of Chemical Bond Formed When There Is A Complete Transfer Of Valence Electrons From One.
Web up to $3 cash back get the detailed answer: The two most basic types of. Web up to 6% cash back sodium will react with oxygen and form an ionic compound. Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond?
Web Which Element Reacts With Oxygen To Form Ionic Bonds?
The electronegativity of oxygen is 3.5 therefore any of the alkali or alkaline metals will ionically bond with oxygen to form an ionic compound. Web which element reacts with oxygen to form ionic bonds? A lithium atom will lose 1 electron to form a stable 1 + ion. (1) calcium (3) chlorine (2) hydrogen (4) nitrogen.
There Are General Trends In The Reactions Between Main Group Elements And Oxygen:
Web according to the electronic configuration, elements with 1 or 2 valence electrons react with oxygen to form ionic bonds.what is electronic configuration?electronic The magnesium would lose two electrons,. 8 months, 2 weeks ago. Web the positive/negative charge attraction would hold the two ions together.
35 Which Element Reacts With Oxygen To Form Ionic Bonds?
35 which element reacts with oxygen to form ionic bonds? Lithium is in group 1 of the periodic table. (1) calcium (3) chlorine (2) hydrogen (4) nitrogen. Gains electrons and its radius gets.