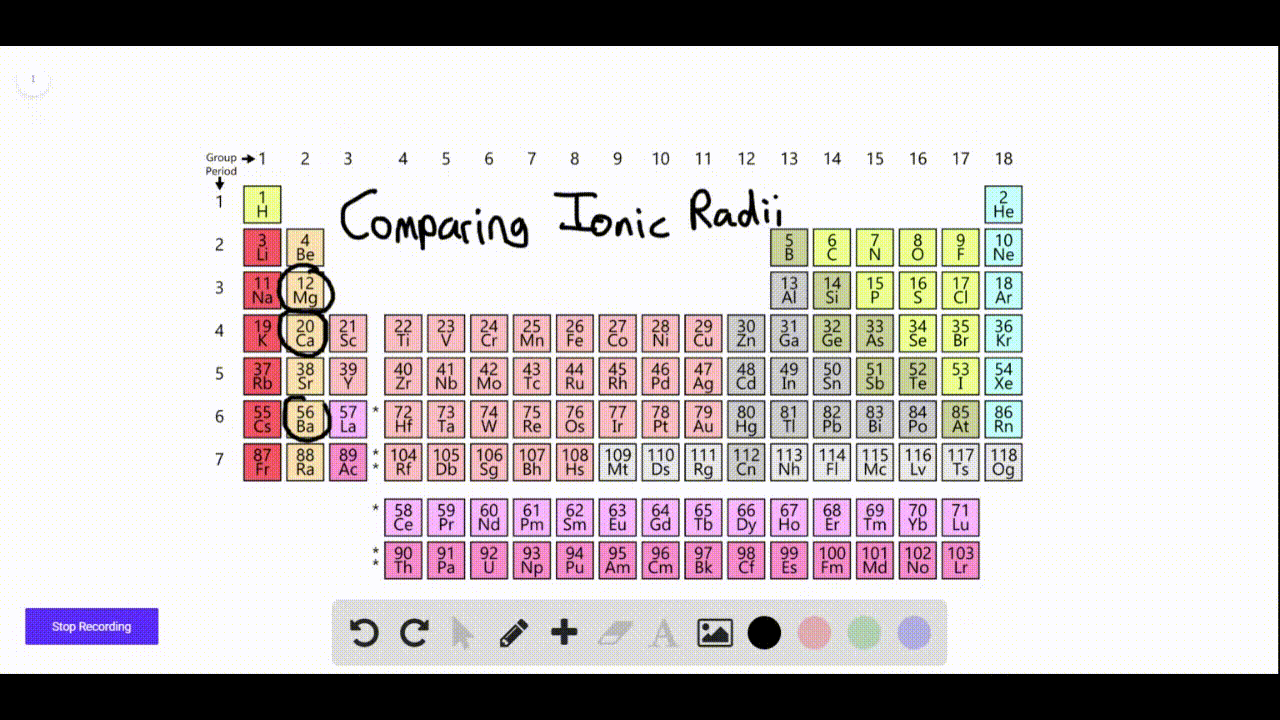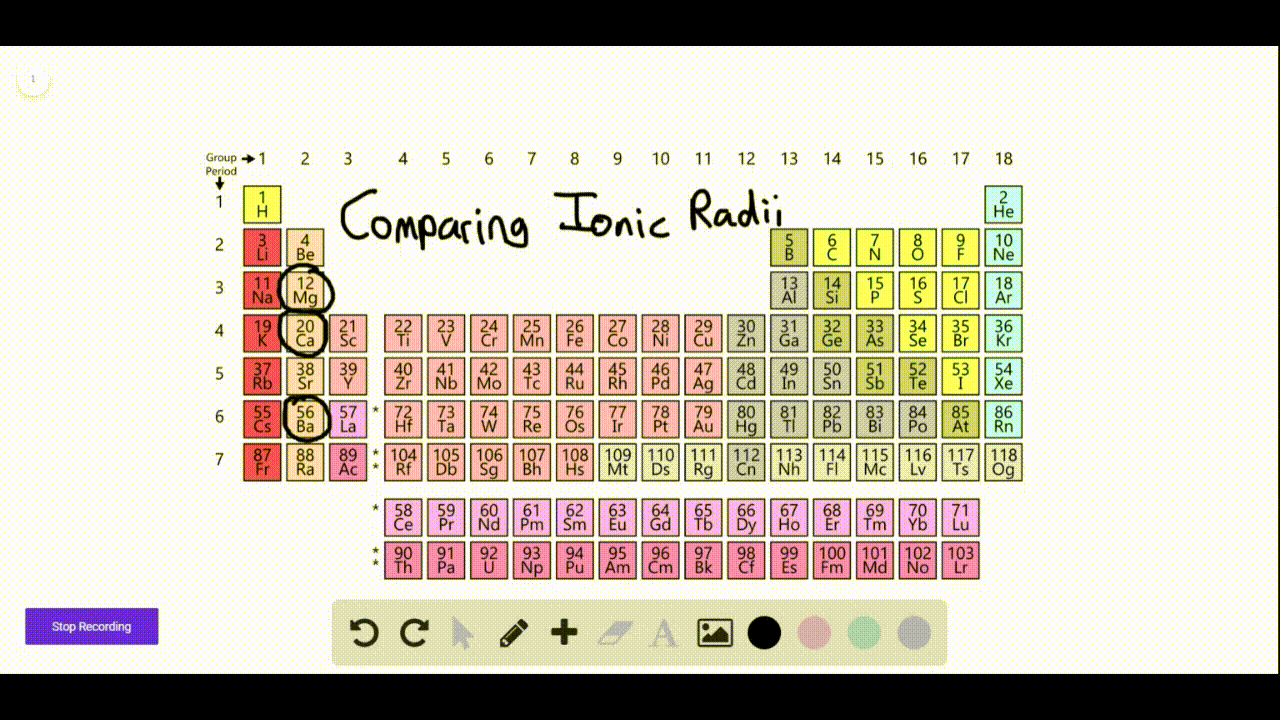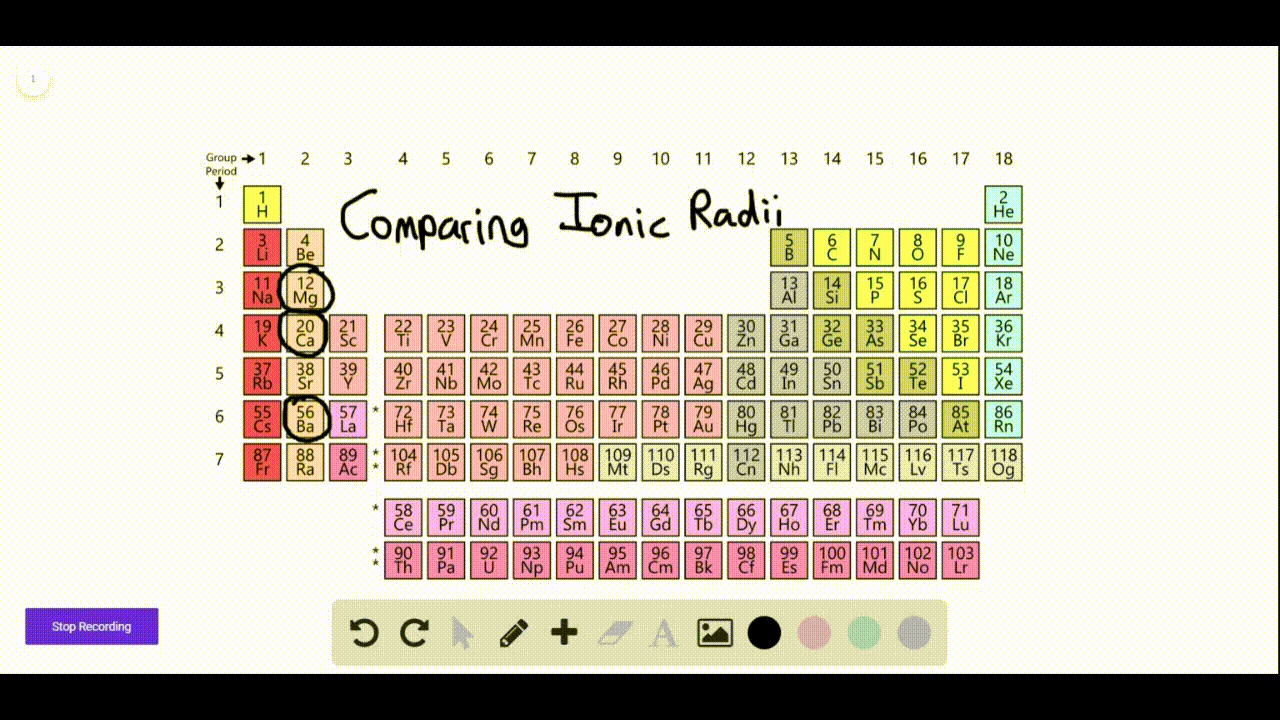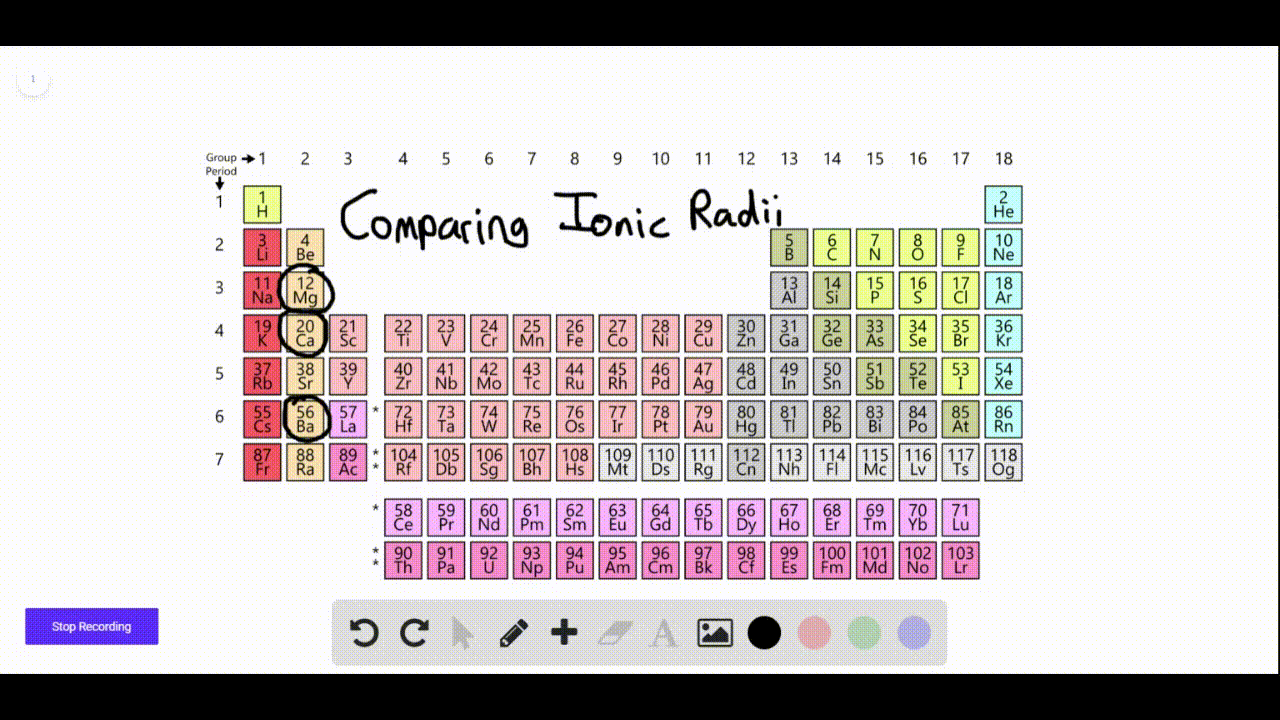
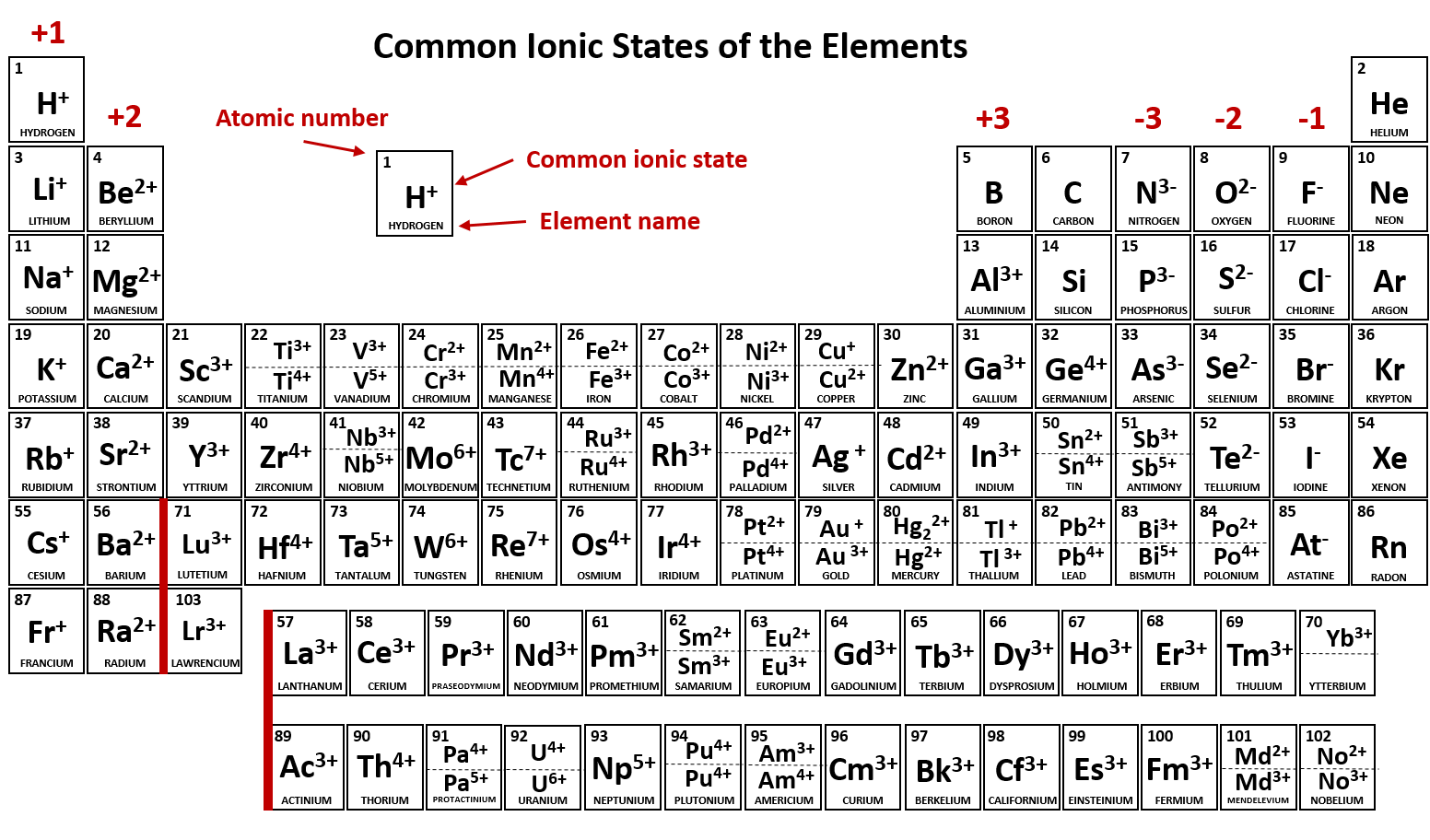
Which Elements Form Negative Ions
Which Elements Form Negative Ions - Web it is going to have six electrons and that's what makes it neutral. The protons do not change. Oxygen is in group 6. Now you could have a carbon. The parent atoms are oxygenating species: Web 5 rows forming negative ions. Electrons have a negative charge, whereas. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. You have the six positive charges and the six negative charges. When nonmetal atoms gain electrons, they often do so until their outermost principal.
Naming an ion is straightforward. The right hand side of the periodic table as we face it. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Use lewis diagrams to illustrate ion formation. Web ions are formed as a result of this addition or removal of electrons from the atom. Produce negative ions, such as oxygen. The parent atoms are oxygenating species: Oxygen is in group 6. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Electrons have a negative charge, whereas.
Web forming negative ions (anions) atoms gain electrons in their outer shell when they form. The right hand side of the periodic table as we face it. The ions formed are negative, because they have more electrons than protons Negative ions are called anions. Positively charged ions are called cations; Web in this way, nitrogen, oxygen, fluorine, chlorine, etc., are formed. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Use lewis diagrams to illustrate ion formation. You have the six positive charges and the six negative charges. When nonmetal atoms gain electrons, they often do so until their outermost principal.
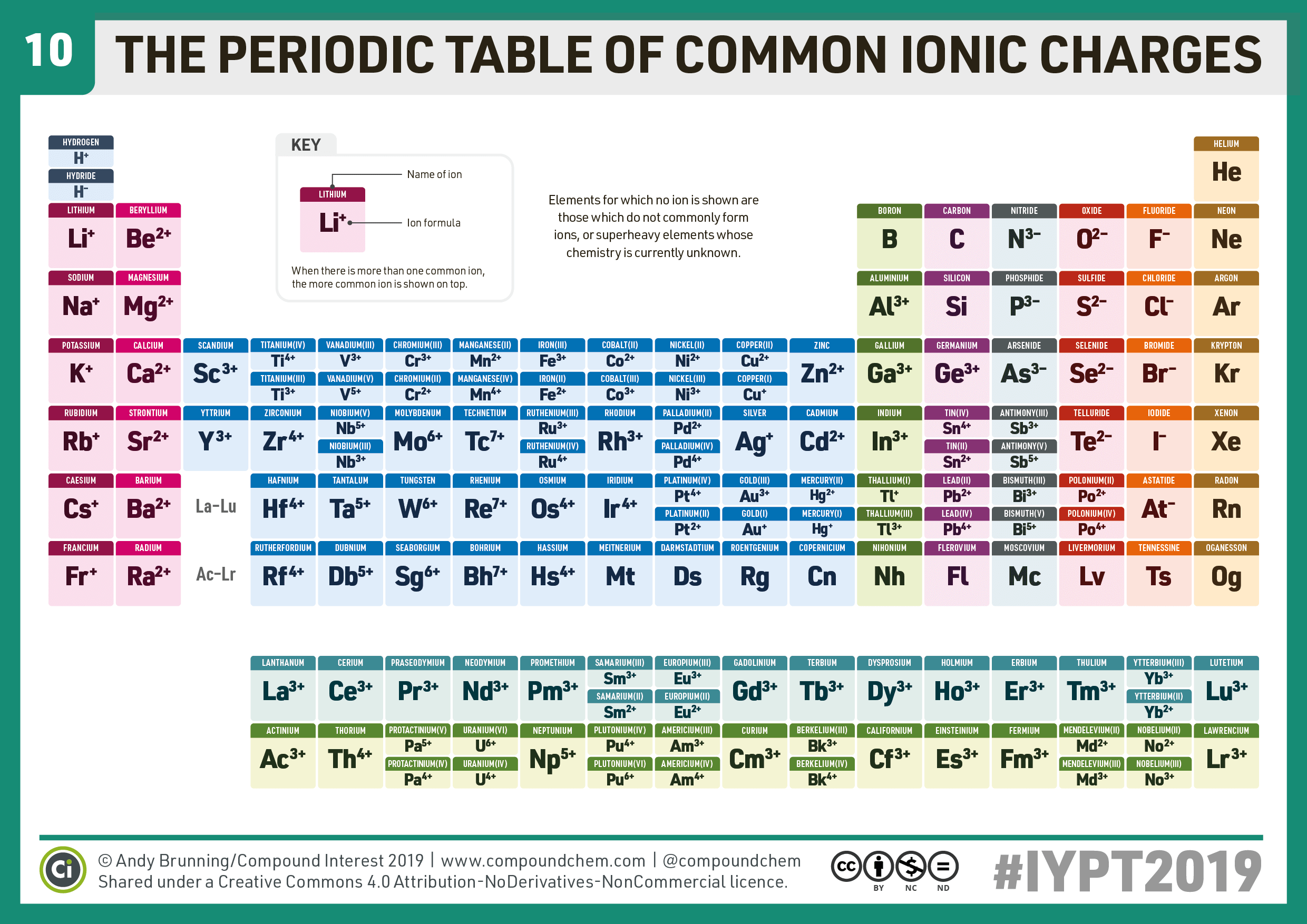
10 Periodic Table of Common Ions Compound Interest
The ions formed are negative, because they have more electrons than protons Web in this way, nitrogen, oxygen, fluorine, chlorine, etc., are formed. When nonmetal atoms gain electrons, they often do so until their outermost principal. Web the elements that would form negative ions the easiest are the halogens. Web moving from the far right to the left on the.
The Chemistry of Ion Exchange WCP Online
Web 5 rows forming negative ions. Web the elements that would form negative ions the easiest are the halogens. Web in this way, nitrogen, oxygen, fluorine, chlorine, etc., are formed. Ionic compounds form as electrons move from metal atoms to. You have the six positive charges and the six negative charges.
10 Best Printable Periodic Table Of Ions
Most atoms do not have eight electrons in their valence electron. Positively charged ions are called cations; Electrons have a negative charge, whereas. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Web 5 rows forming.
Electron Affinity of The Elements
Produce negative ions, such as oxygen. Web an ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons. The protons do not change. The parent atoms are oxygenating species: Web forming negative ions (anions) atoms gain electrons in their outer shell when they form.
3.2 Ions The Basics of General, Organic, and Biological Chemistry
The right hand side of the periodic table as we face it. Web anions are the negative ions formed from the gain of one or more electrons. Produce negative ions, such as oxygen. Use lewis diagrams to illustrate ion formation. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound.
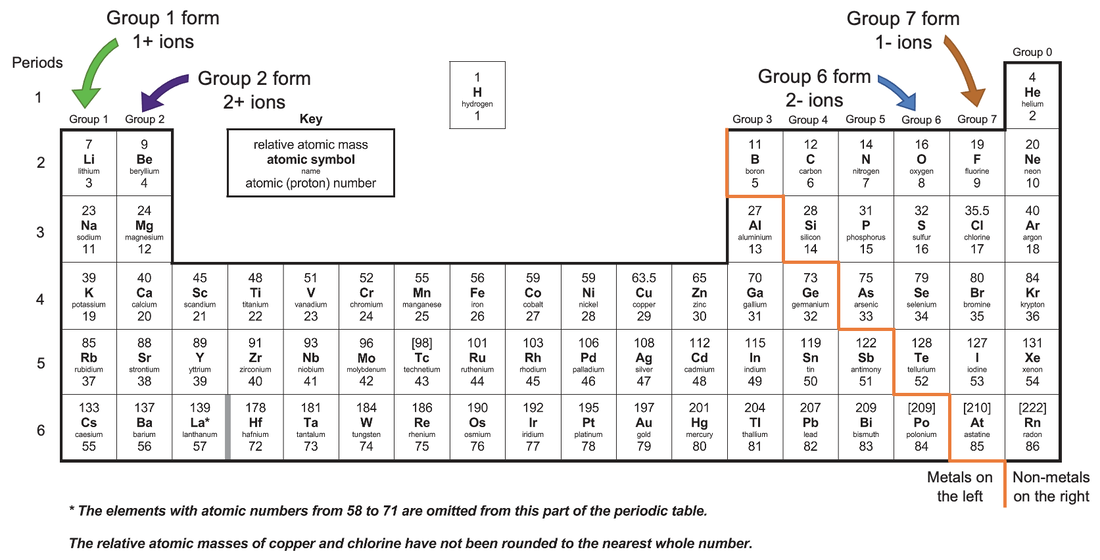
C2 B) Ions from the Periodic Table AQA Combined Science Trilogy Elevise
Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Now you could have a carbon. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Electrons have a negative charge, whereas. Web learning objectives define the two types of ions.
SOLVEDAn element forms a negative ion when ionized. On what side of
Negative ions are called anions. The parent atoms are oxygenating species: When nonmetal atoms gain electrons, they often do so until their outermost principal. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Web ion, any.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. The parent atoms are oxygenating species: Web 5 rows forming negative ions. When nonmetal atoms gain electrons, they often do so until their outermost principal. Produce negative.
Ions
Web ions are formed as a result of this addition or removal of electrons from the atom. The ions formed are negative, because they have more electrons than protons Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Use lewis diagrams to illustrate ion formation. Negative ions, known as anions, form.
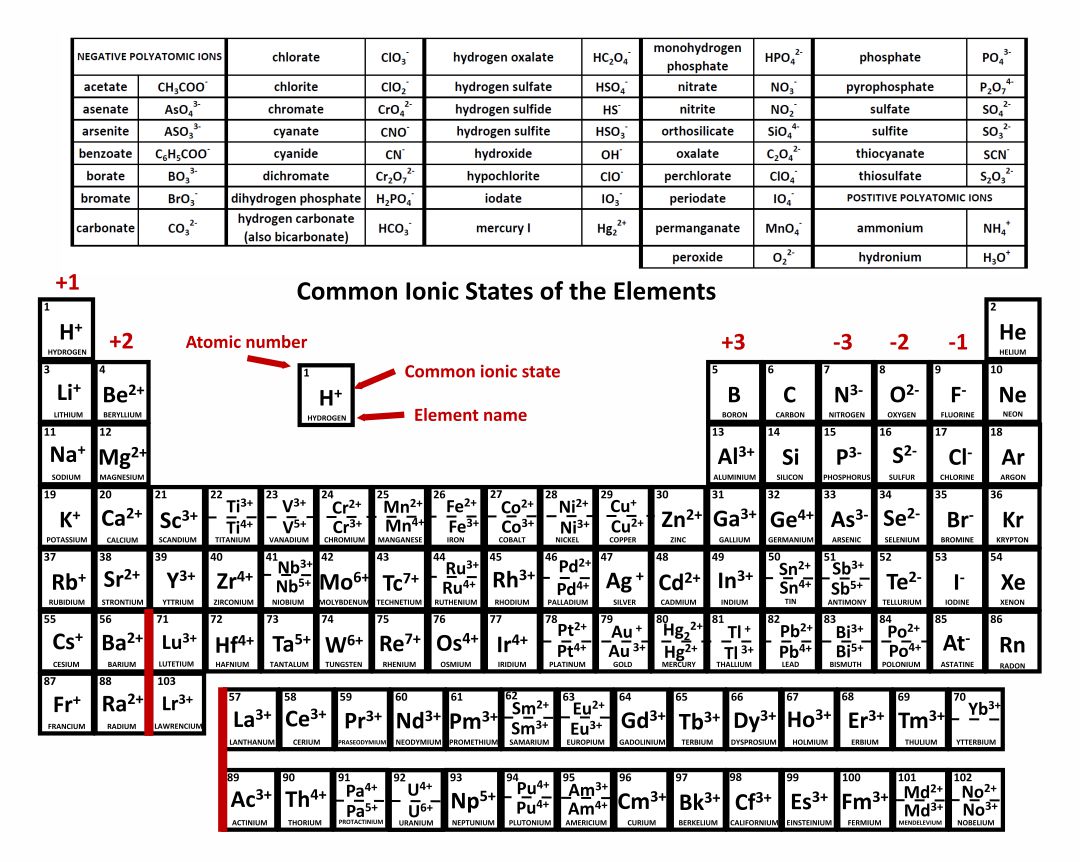
Periodic Table Ionic Charges Pdf Awesome Home
Web learning objectives define the two types of ions. The protons do not change. Web ions are formed as a result of this addition or removal of electrons from the atom. When nonmetal atoms gain electrons, they often do so until their outermost principal. Produce negative ions, such as oxygen.
Web An Ionic Bond Is An Electrostatic Attraction Between The Positive And Negative Ions Of A Chemical Compound.
Web learning objectives define the two types of ions. Electrons have a negative charge, whereas. The right hand side of the periodic table as we face it. These are the elements with 7 valence electrons.
You Have The Six Positive Charges And The Six Negative Charges.
Produce negative ions, such as oxygen. Because of this configuration, they will. Web it is going to have six electrons and that's what makes it neutral. Web a negative ion is formed by the addition of negatively charged electrons.
Positively Charged Ions Are Called Cations;
The protons do not change. When nonmetal atoms gain electrons, they often do so until their outermost principal. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. The parent atoms are oxygenating species:
Web In This Way, Nitrogen, Oxygen, Fluorine, Chlorine, Etc., Are Formed.
Web an ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons. Most atoms do not have eight electrons in their valence electron. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Oxygen is in group 6.