Which Pair Of Elements Can Form An Ionic Compound
Which Pair Of Elements Can Form An Ionic Compound - Therefore, one lithium (li) cation bonds with one fluorine (f) anion as lithium. For example, cabr 2 contains a metallic element (calcium, a group 2 [or 2a]. Web science chemistry chemistry questions and answers part a determine whether the following pairs of elements can form ionic compounds. Web which pair of elements can form an ionic compound with each other? Select the correct answer below: C and s c and o. Nitrogen, hydrogern 011, wht this problem has. Which pair of elements will form an ionic compound? Compounds between metal and nonmetal elements are usually ionic. As you can see, there are no.
Write the symbol for each. The alkali halides (nacl, lif, etc.). Therefore, one lithium (li) cation bonds with one fluorine (f) anion as lithium. Web molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. 4/28/2022 wiki user ∙ 10y ago study now see answer (1) best answer copy usually, a metallic. Web which pair of elements form an ionic compound? Web aluminum and carbon react to form an ionic compound. They can conduct electricity and are usually highly water. Web determine whether the following pairs of elements can form ionic compounds. Web which pair of elements can form an ionic compound with each other?
For example, cabr 2 contains a metallic element (calcium, a group 2 [or 2a]. Web aluminum and carbon react to form an ionic compound. Web molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The periodic table ( figure 3.2 “a simple periodic. As you can see, there are no. Write the symbol for each. Web which pair of elements can form an ionic compound with each other? C and s c and o. Which pair of elements will form an ionic compound? Web determine whether the following pairs of elements can form ionic compounds.
Periodic Table Ions List Periodic Table Timeline
Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. 4/28/2022 wiki user ∙ 10y ago study now see answer (1) best answer copy usually, a metallic. Which pair of elements will form an ionic compound? As you can see, there are no. Web molecular compounds form between.
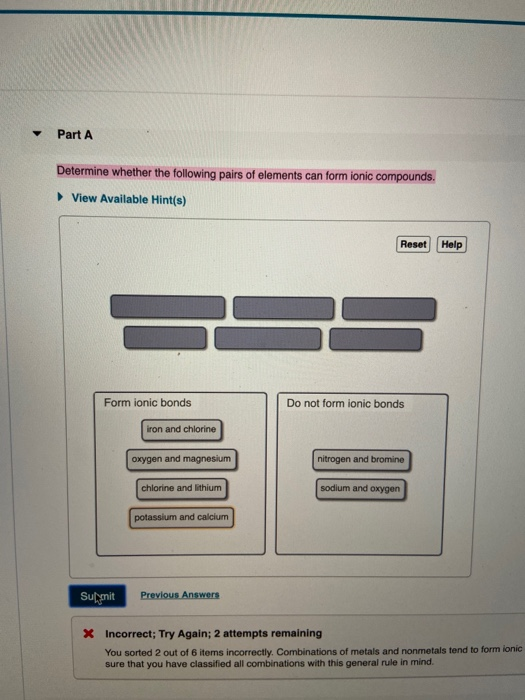
Solved Part A Determine whether the following pairs of
It is formed when there is a transfer of the valence electron of the atoms generating two oppositely. C and s c and o. Select the correct answer below: Write the symbol for each. Web aluminum and carbon react to form an ionic compound.
4.1.6 Predict if a compound of 2 elements is ionic using the table of
Web molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal. They can conduct electricity and are usually highly water. Select the correct answer below: The periodic table ( figure 3.2 “a simple periodic.
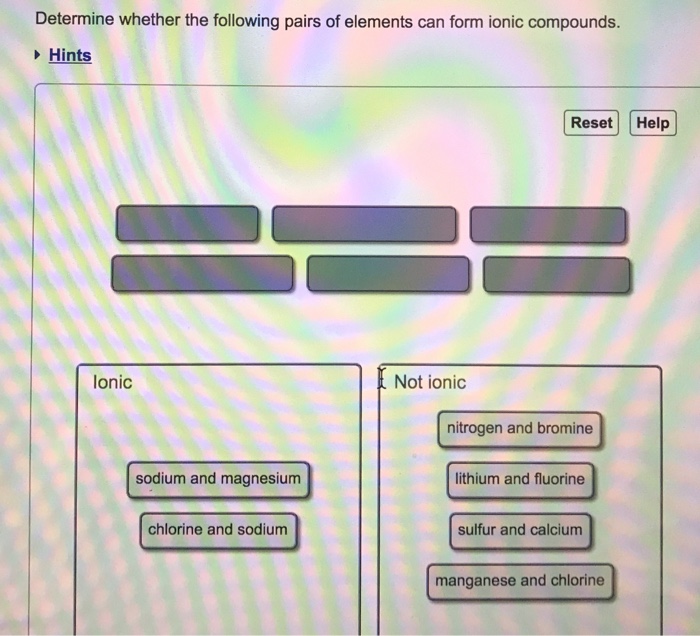
Solved Determine Whether The Following Pairs Of Elements
The periodic table ( figure 3.2 “a simple periodic. Write the symbol for each. Web which pair of elements can form an ionic compound with each other? Web aluminum and carbon react to form an ionic compound. It is formed when there is a transfer of the valence electron of the atoms generating two oppositely.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
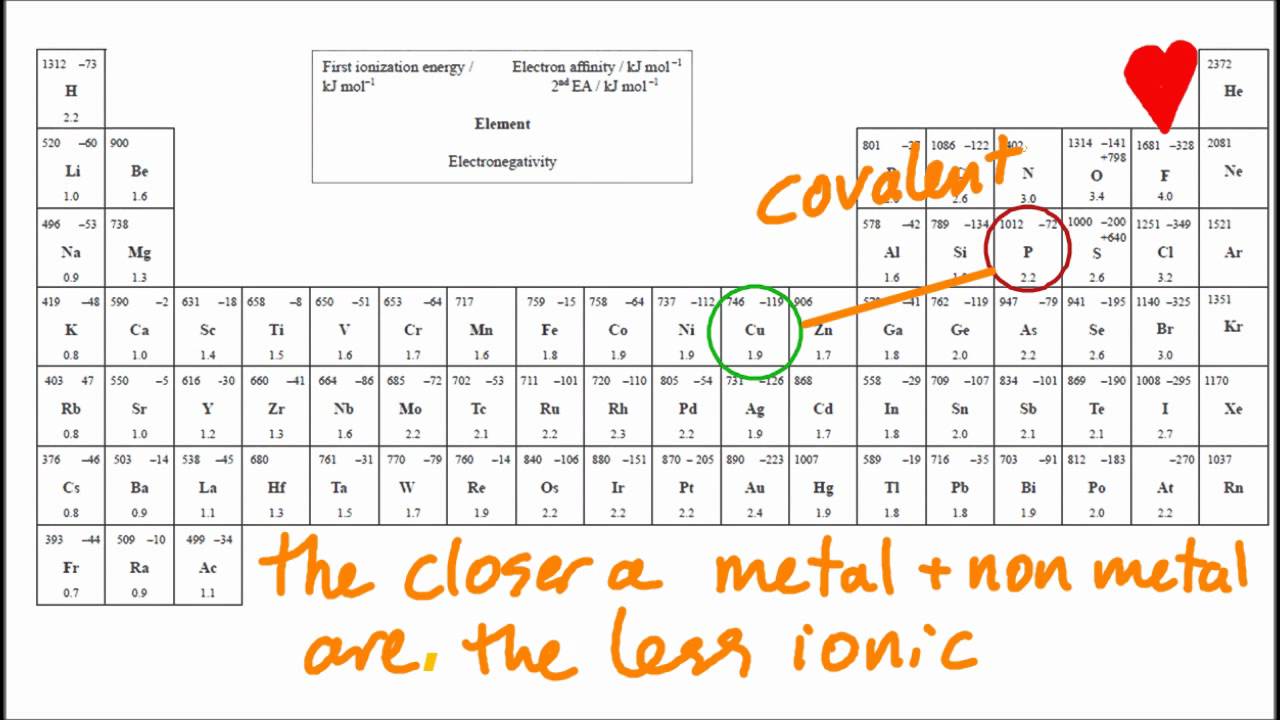
The periodic table ( figure 3.2 “a simple periodic. For example, cabr 2 contains a metallic element (calcium, a group 2 [or 2a]. Compounds between metal and nonmetal elements are usually ionic. Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal. Web the elements that tend to form ionic compounds include.
Chemistry Archive January 09, 2017
Web aluminum and carbon react to form an ionic compound. Compounds between metal and nonmetal elements are usually ionic. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. Web which.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web which pair of elements can form an ionic compound with each other? Write the symbol for each. The periodic table ( figure 3.2 “a simple periodic. Therefore, one lithium (li) cation bonds with one fluorine (f) anion as lithium. Select the correct answer below:
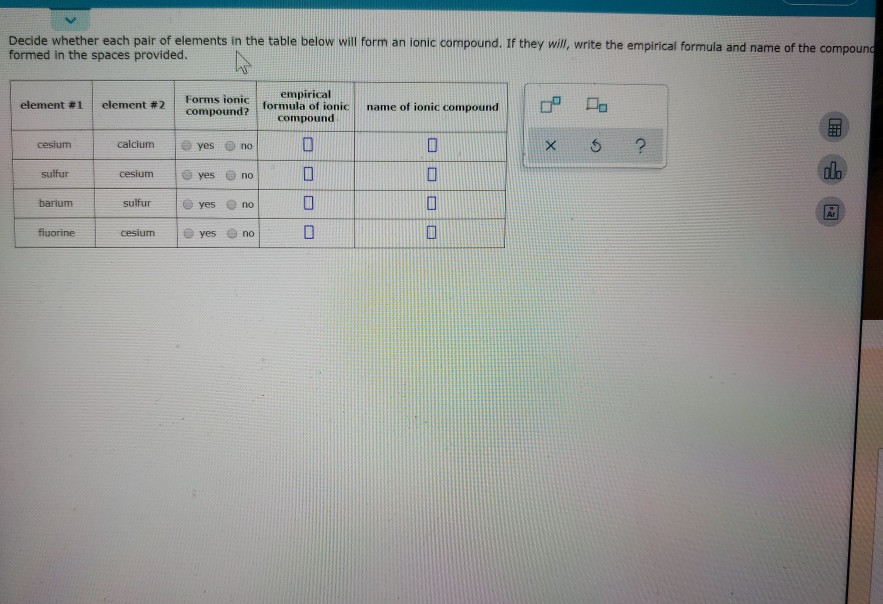
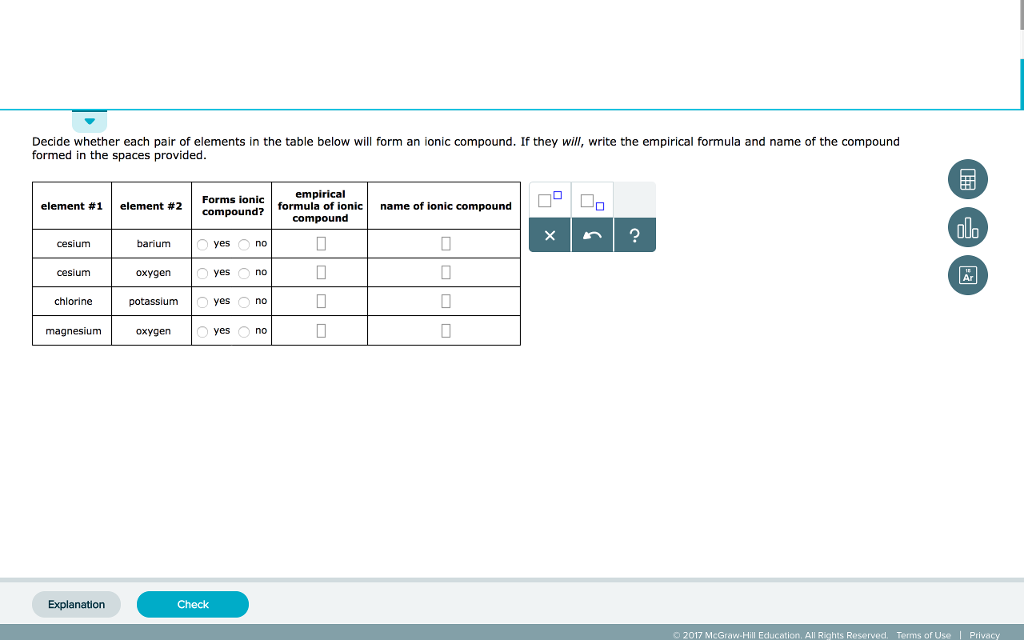
Solved Decide whether each pair of elements in the table
Web aluminum and carbon react to form an ionic compound. For example, cabr 2 contains a metallic element (calcium, a group 2 [or 2a]. Therefore, one lithium (li) cation bonds with one fluorine (f) anion as lithium. 4/28/2022 wiki user ∙ 10y ago study now see answer (1) best answer copy usually, a metallic. Web ionic bonding occurs in compounds.
Naming Simple Ionic Compounds Pathways to Chemistry
Web which pair of elements can form an ionic compound with each other? Nitrogen, hydrogern 011, wht this problem has. Web which pair of elements form an ionic compound? The correct option is b) one atom of sodium and one atom of fluorine. Sodium is a metal with a low electronegativity it will form an ionic bond with a non.
Periodic Table With Charges Printable 29 Printable Periodic Tables
Select the correct answer below: The periodic table ( figure 3.2 “a simple periodic. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. Therefore, one lithium (li) cation bonds with one fluorine (f) anion as lithium. Web chemistry 1 answer david drayer jun 6, 2018 n af n a2s explanation:
Sodium Is A Metal With A Low Electronegativity It Will Form An Ionic Bond With A Non Metal.
The alkali halides (nacl, lif, etc.). Web aluminum and carbon react to form an ionic compound. Web determine whether the following pairs of elements can form ionic compounds. Compounds between metal and nonmetal elements are usually ionic.
The Correct Option Is B) One Atom Of Sodium And One Atom Of Fluorine.
Therefore, one lithium (li) cation bonds with one fluorine (f) anion as lithium. The periodic table ( figure 3.2 “a simple periodic. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. For example, cabr 2 contains a metallic element (calcium, a group 2 [or 2a].
Web Chemistry 1 Answer David Drayer Jun 6, 2018 N Af N A2S Explanation:
They can conduct electricity and are usually highly water. It is formed when there is a transfer of the valence electron of the atoms generating two oppositely. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. Nitrogen, hydrogern 011, wht this problem has.
Web Ionic Bonding Occurs In Compounds Composed Of Strongly Electropositive Elements (Metals) And Strongly Electronegative Elements (Nonmetals).
Web science chemistry chemistry questions and answers part a determine whether the following pairs of elements can form ionic compounds. Web which pair of elements form an ionic compound? C and s c and o. Predict which forms an anion, which forms a cation, and the charges of each ion.


.PNG)