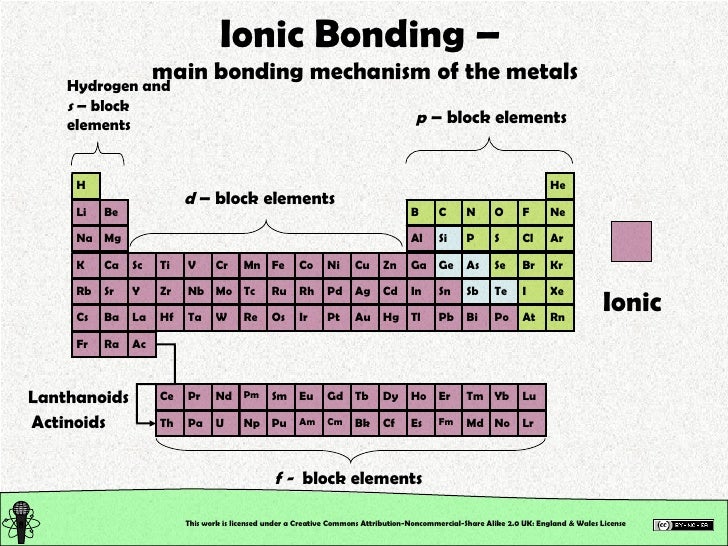
Which Pair Of Elements Form An Ionic Bond
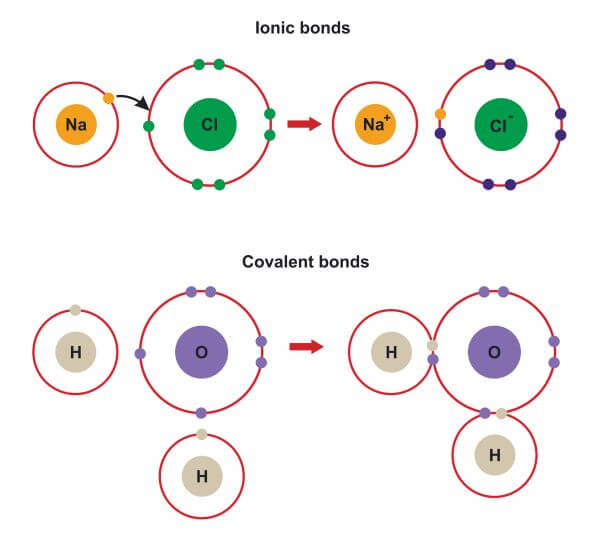
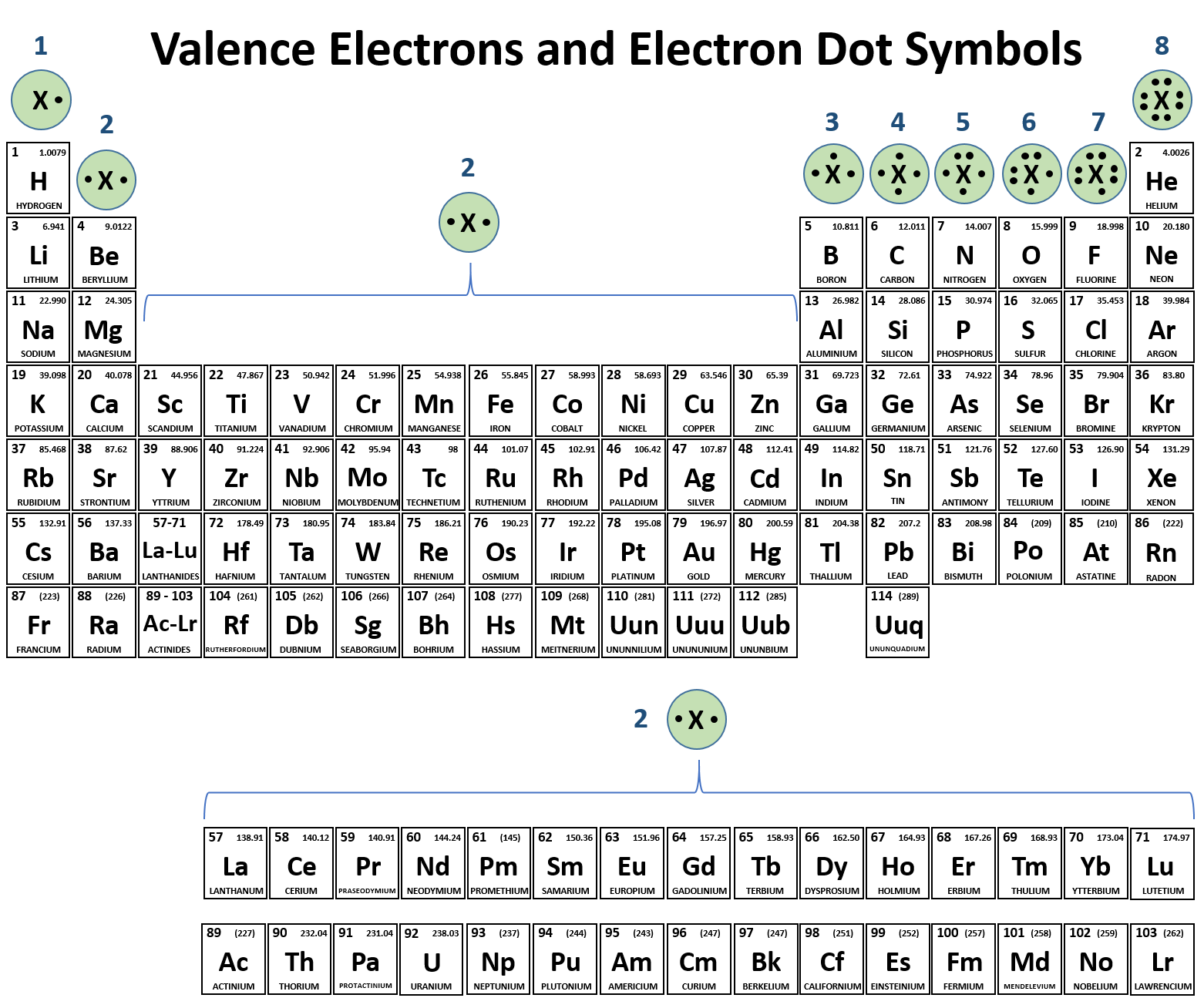
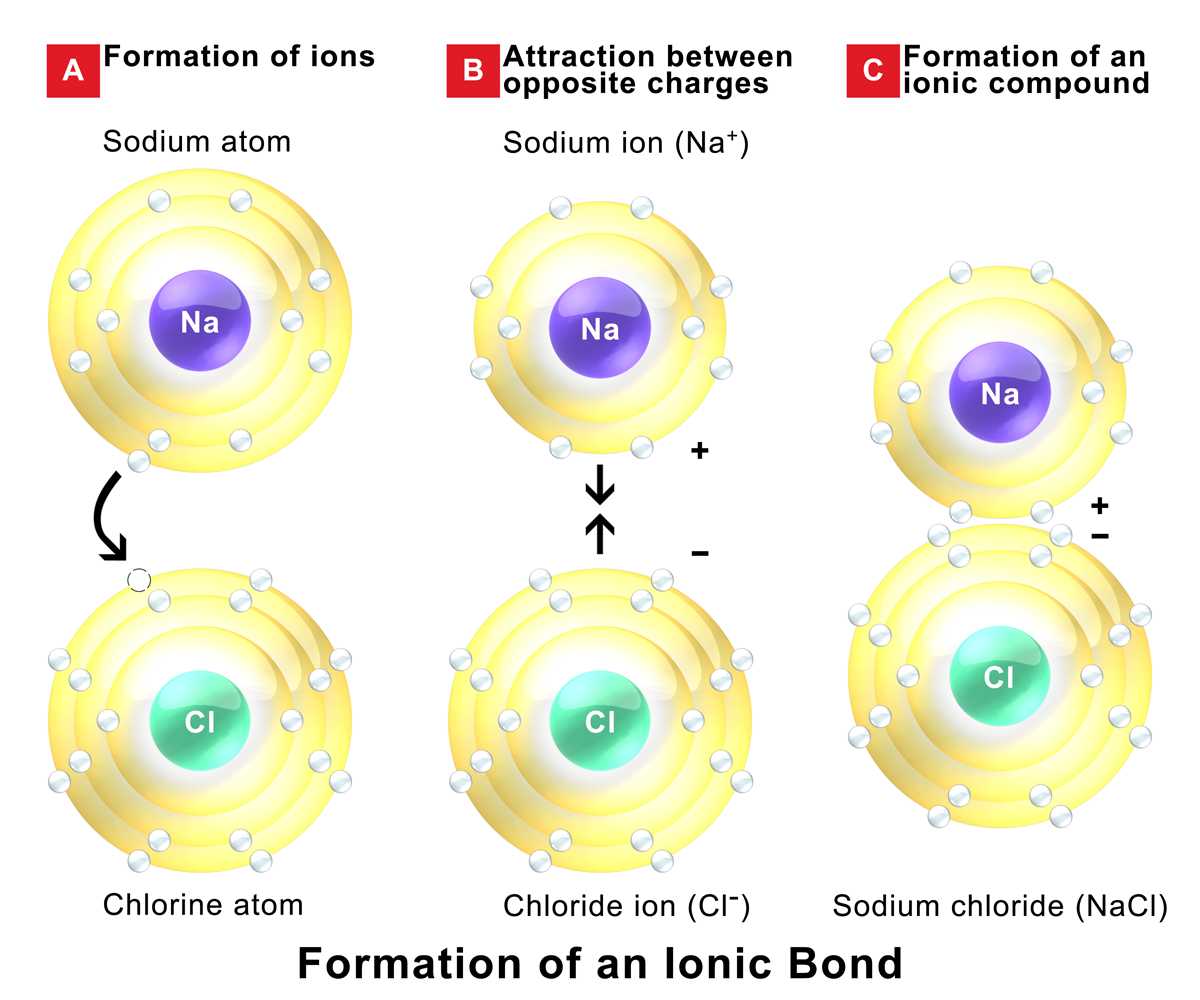
Which Pair Of Elements Form An Ionic Bond - Web which pair of elements would form an ionic bond? The combination of these ions form in numerical combinations that generate a neutral (zero charge). Web ethene contains a carbon/carbon double bond. See answers advertisement advertisement brainly user brainly user answer: An ionic bond is formed between compounds with a large electronegativity difference between them. Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Following this pattern, the triple bond in ethyne molecular formula c 2 h 2, (also known as acetylene, the fuel used in. Ionic bond is formed by complete transfer of electron/s by an atom of electropositive. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal with a high electronegativity.
Web 1, which pair of elements is most likely to form an ionic bond. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). Web answer 52 people found it helpful podgorica option (2) strontium and chlorine. Web comparison of ionic and covalent bonds. Web ionic bonds are formed by the combination of positive and negative ions; Web ethene contains a carbon/carbon double bond. Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. An ionic bond is formed between compounds with a large electronegativity difference between them. Web which elements form ionic bonds? Both florine and sulfur are non.
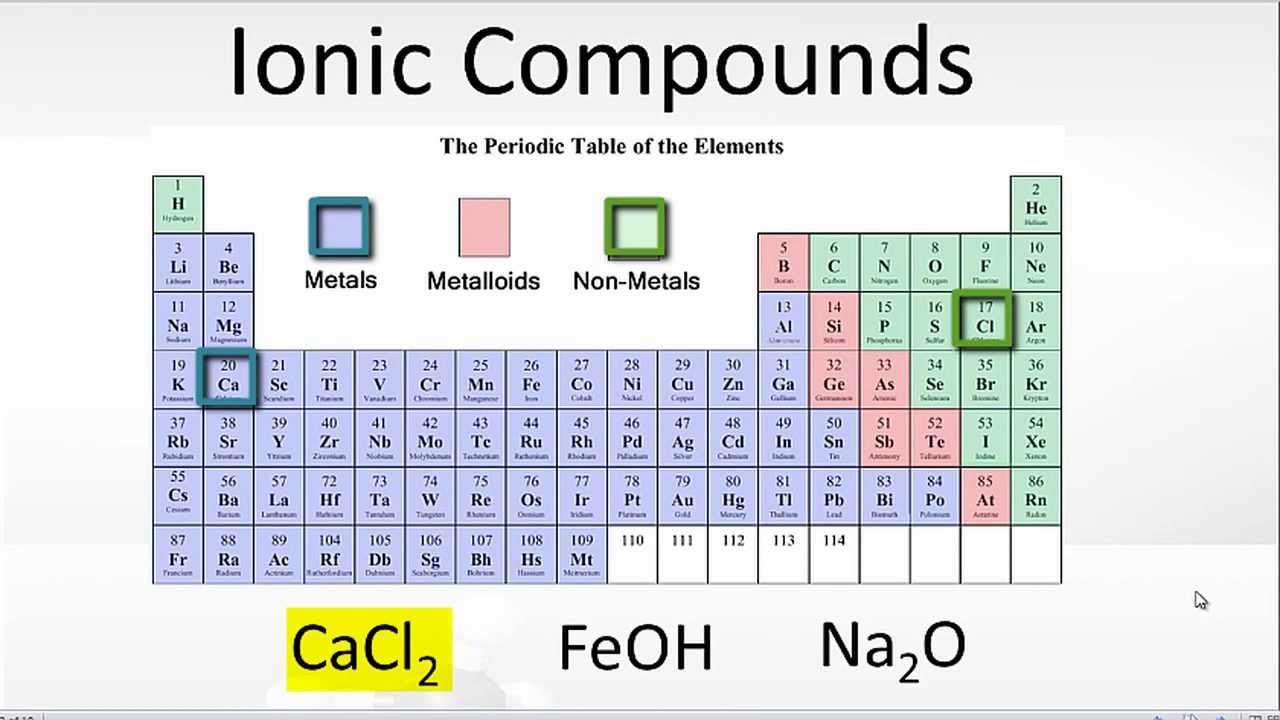
Web which pair of elements would form an ionic bond? For example, cabr 2 contains a metallic element (calcium, a group 2a metal) and a nonmetallic. Web the pair of elements which on combination are most likely to form an ionic compound is: Web which pair of elements will form an ionic bond? Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). In general, covalent bonds form. Web comparison of ionic and covalent bonds. Both florine and sulfur are non. See answers advertisement advertisement brainly user brainly user answer: Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals).
Naming Simple Ionic Compounds Pathways to Chemistry
Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web 1, which pair of elements is most likely to form an ionic bond. Web first, compounds between metal and nonmetal elements are usually ionic. Web ionic bonds are formed by the combination of positive and negative ions; Web which pair of elements.
Ionic Bond Definition, Types, Properties & Examples
One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Web ethene contains a carbon/carbon double bond. Web which pair of elements will form an ionic bond? Web the pair of elements which on combination are most likely to form an ionic compound is: Web ionic.
Ionic Bond Examples Biology Dictionary
Carbon (c) and oxygen (o) b. An ionic bond is formed between compounds with a large electronegativity difference between them. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). In general, covalent bonds form. Cesium (cs) and germanium (ge) d.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
An ionic bond is formed between compounds with a large electronegativity difference between them. In general, covalent bonds form. See answers advertisement advertisement brainly user brainly user answer: Ionic bond is formed by complete transfer of electron/s by an atom of electropositive. Web ethene contains a carbon/carbon double bond.
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
Ionic bond is formed by complete transfer of electron/s by an atom of electropositive. Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Web which pair of elements will form an ionic bond? Web 1, which pair of elements is most.
Periodic Table Ions List Periodic Table Timeline
Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web the pair of elements which on combination are most likely to form an ionic compound is: Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic bond is formed by complete transfer of electron/s by an atom of electropositive. Web which pair of elements would form an ionic bond? For example, cabr 2 contains a metallic element (calcium, a group 2a metal) and a nonmetallic. Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull.
Examples of Ionic Bonds and Ionic Compounds
Web ethene contains a carbon/carbon double bond. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web the pair of elements which on combination are most likely to form an ionic compound is: The combination of these ions form in numerical combinations that generate a neutral (zero charge). Web first, compounds between.
Chemical Structure Chemical Bonding. Ionic, Metallic & Coordinate Bo…
Potassium (k) and bromine (br) explanation: Ionic bond is formed by complete transfer of electron/s by an atom of electropositive. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). As we have seen, there are. In general, covalent bonds form.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web comparison of ionic and covalent bonds. Web first, compounds between metal and nonmetal elements are usually ionic. Web ethene contains a carbon/carbon double bond. Web ionic bonds are formed by the combination of positive and negative ions; Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals).
See Answers Advertisement Advertisement Brainly User Brainly User Answer:
Ionic bond is formed by complete transfer of electron/s by an atom of electropositive. Strontium (sr) and chlorine (cl) c. Carbon (c) and oxygen (o) b. Both florine and sulfur are non.
Web First, Compounds Between Metal And Nonmetal Elements Are Usually Ionic.
Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal with a high electronegativity. Web comparison of ionic and covalent bonds. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). Web 1, which pair of elements is most likely to form an ionic bond.
The Combination Of These Ions Form In Numerical Combinations That Generate A Neutral (Zero Charge).
Web ionic bonds are formed by the combination of positive and negative ions; Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. In general, covalent bonds form. A molecule or compound is made when two or more atoms form a chemical bond that links them together.
Web Ethene Contains A Carbon/Carbon Double Bond.
Following this pattern, the triple bond in ethyne molecular formula c 2 h 2, (also known as acetylene, the fuel used in. Web which elements form ionic bonds? An ionic bond is formed between compounds with a large electronegativity difference between them. Web the pair of elements which on combination are most likely to form an ionic compound is:



/ionic-bond-58fd4ea73df78ca1590682ad.jpg)
.PNG)