Which Pair Of Elements Will Form A Covalent Bond
Which Pair Of Elements Will Form A Covalent Bond - Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Some compounds contain both covalent and ionic bonds. Web covalent bonds are formed only when the electron pair is shared between two atoms. Web two different atoms can also share electrons and form covalent bonds. Web which pair of elements will form a covalent bond? Two electrons or one electron pair constitute a single covalent bond in. Some compounds contain both covalent and ionic bonds. Web a covalent bond is formed by the equal sharing of electrons from both participating atoms. Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. See answer advertisement advertisement brainly user brainly user hydrogen and chlorine.
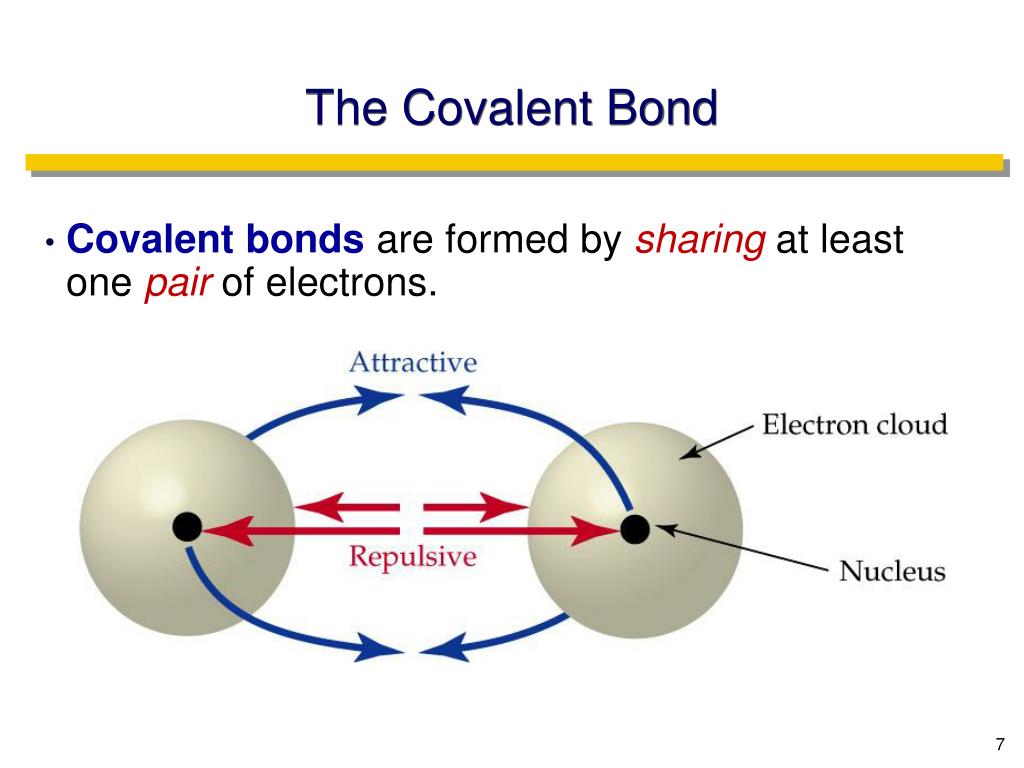
Some compounds contain both covalent and ionic bonds. Chemical compound is a combination of molecule,. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web compounds can be covalent or ionic. The classification of covalent bonds is done in three ways, depending on the no. Web a covalent bond consists of the simultaneous attraction of two nuclei for one or more pairs of electrons. Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. Web two different atoms can also share electrons and form covalent bonds. Web covalent bonds are formed only when the electron pair is shared between two atoms. These electron pairs are known as shared pairs or bonding.
Web compounds can be covalent or ionic. An atom that shares one or more of its. Web two different atoms can also share electrons and form covalent bonds. See answer advertisement advertisement brainly user brainly user hydrogen and chlorine. Web a covalent bond consists of the simultaneous attraction of two nuclei for one or more pairs of electrons. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Covalent bonds form only between atoms of. Web a covalent bond is formed by the equal sharing of electrons from both participating atoms. Web a covalent bond is the force of attraction that holds together two atoms that share a pair of valence electrons. Web in order to form a covalent bond, each element has to share one unpaired electron.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web ionic bonds require at least one electron donor and one electron acceptor. Web in order to form a covalent bond, each element has to share one unpaired electron. Web bonds between two nonmetals are generally covalent; Web which pair of atoms will form a covalent bond? Covalent bonds form only between atoms of.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Covalent bonds form only between atoms of. The electrons located between the two nuclei are bonding electrons. Web a covalent bond is formed by the equal sharing of electrons from both participating atoms. In contrast, atoms with the same electronegativity share electrons in covalent bonds,. The single electrons match up to make pairs (fig.
PPT Covalent Bonding PowerPoint Presentation, free download ID4003633
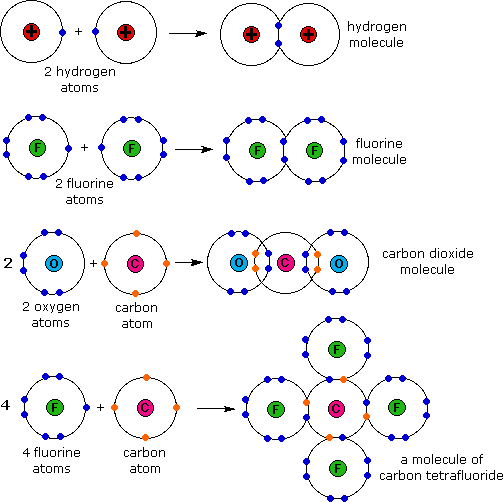
Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. Web which pair of atoms will form a covalent bond? Web covalent bond is formed by sharing of electron. Web two different atoms can also share electrons and form covalent.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
The single electrons match up to make pairs (fig. Web types of covalent bonds. The pair of electrons participating in this type of bonding is called a shared pair or. Web which pair of atoms will form a covalent bond? The electrons located between the two nuclei are bonding electrons.
2.2 Chemical Bonds Anatomy & Physiology
Some compounds contain both covalent and ionic bonds. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web in order to form a covalent bond, each element has to share one unpaired electron. See answer advertisement advertisement brainly user brainly user hydrogen and chlorine. The classification of covalent bonds.
ASSTUDYPEACH Covalent Bonds Sharing Is Caring!
Some compounds contain both covalent and ionic bonds. The single electrons match up to make pairs (fig. Bonding between a metal and a nonmetal is often ionic. Web compounds can be covalent or ionic. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals).
Covalent Bond Examples Several Examples of Covalent (molecular) Bonds
In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Bonding between a metal and a nonmetal is often ionic. The oxygen atom forms two. Covalent bonds form only between atoms of. Web a covalent bond consists of the simultaneous attraction of two nuclei for one or more pairs of electrons.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Web covalent bonds are formed only when the electron pair is shared between two atoms. Two electrons or one electron pair constitute a single covalent bond in. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. An atom that shares one or more of its. Web a covalent bond is formed.
Covalent Bonding (Biology) — Definition & Role Expii
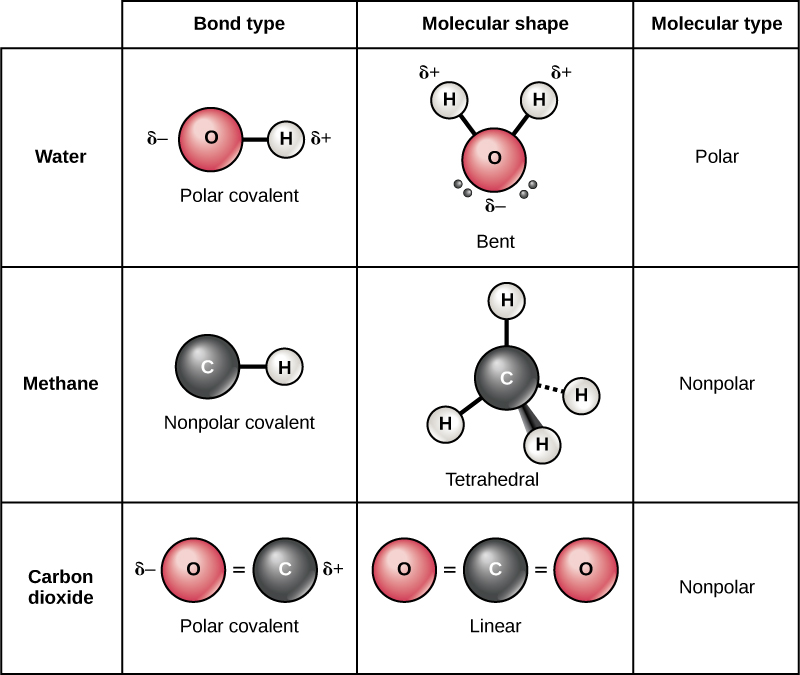
Two electrons or one electron pair constitute a single covalent bond in. Web bonds between two nonmetals are generally covalent; Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom. The classification of covalent bonds.
IGCSE Chemistry 2017 1.44 Know that a Covalent Bond is Formed Between
Web covalent bonds are formed only when the electron pair is shared between two atoms. Bonding between a metal and a nonmetal is often ionic. Some compounds contain both covalent and ionic bonds. See answer advertisement advertisement brainly user brainly user hydrogen and chlorine. The classification of covalent bonds is done in three ways, depending on the no.
Web A Covalent Bond Is A Chemical Bond That Involves The Sharing Of Electrons To Form Electron Pairs Between Atoms.
The electrons located between the two nuclei are bonding electrons. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. See answer advertisement advertisement brainly user brainly user hydrogen and chlorine.
Web Ionic Bonds Require At Least One Electron Donor And One Electron Acceptor.
Web bonds between two nonmetals are generally covalent; Web compounds can be covalent or ionic. Web in order to form a covalent bond, each element has to share one unpaired electron. An atom that shares one or more of its.
The Single Electrons Match Up To Make Pairs (Fig.
Molecules of identical atoms, such as h2 and. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Some compounds contain both covalent and ionic bonds. The oxygen atom forms two.
Covalent Bonds Form Only Between Atoms Of.
Web a covalent bond consists of the simultaneous attraction of two nuclei for one or more pairs of electrons. Bonding between a metal and a nonmetal is often ionic. Web which pair of elements will form a covalent bond? For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom.