Ammonium Nitrite Reacts To Form Nitrogen And Water
Ammonium Nitrite Reacts To Form Nitrogen And Water - Web nitrate and ammonium nitrogen are measured for all surface and ground water samples. Web from the reaction is given that the ammonium nitrate ‾ \underline{\text{ammonium nitrate}} ammonium nitrate (n h 4 n o 2) \mathrm{(nh_4no_2)} (n h 4 n o 2 ) reacts to form. Nh → n + 2h. Web ammonia nitrogen is reductive, and the concentration of ammonia nitrogen can be detected indirectly by converting ammonia nitrogen into nitrate or nitrite. Web this reaction mechanism releases the nitrite ion (no2−) to produce an intermediate (nitrosamine (h2nno)) which dissociates very rapidly to form the final. Web ted under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water if 32.7 grams of ammonium nitrite react to form 14.3 grams of. Web interesting facts about ammonium nitrate. Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. These are the most reduced forms of the inorganic. Web ammonium ions and nitrite ions react in water to form nitrogen gas.
If 27.6 grams of ammonium nitrite react to. Web ammonium nitrate is a salt, thus, it easily dissolves in water. Web (c) sodium hydrogen carbonate reacts to form sodium carbonate, water, and carbon dioxide. Web ammonium nitrate decomposes into nitrogen and water? The commercial grade contains about 33.5 percent. Ammonium nitrate is a chemical compound with the formula nh4no3. Web nitrate and ammonium nitrogen are measured for all surface and ground water samples. Web ammonium nitrate, (nh 4 no 3 ), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. Web ted under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water if 32.7 grams of ammonium nitrite react to form 14.3 grams of. Web from the reaction is given that the ammonium nitrate ‾ \underline{\text{ammonium nitrate}} ammonium nitrate (n h 4 n o 2) \mathrm{(nh_4no_2)} (n h 4 n o 2 ) reacts to form.
Web ammonium ions and nitrite ions react in water to form nitrogen gas. Web (c) sodium hydrogen carbonate reacts to form sodium carbonate, water, and carbon dioxide. Nh → n + 2h. Web from the reaction is given that the ammonium nitrate ‾ \underline{\text{ammonium nitrate}} ammonium nitrate (n h 4 n o 2) \mathrm{(nh_4no_2)} (n h 4 n o 2 ) reacts to form. Web nitrate and ammonium nitrogen are measured for all surface and ground water samples. Web if 21.1 grams of ammonium nitrite react to form 9.2 grams of nitrogen, how many grams of water must simultaneously be formed? The decomposition reaction is nh4no3. If 27.6 grams of ammonium nitrite react to. Ammonium nitrate is a chemical compound with the formula nh4no3. Web this reaction mechanism releases the nitrite ion (no2−) to produce an intermediate (nitrosamine (h2nno)) which dissociates very rapidly to form the final.
Ammonium Nitrite, Organic And Solvents Triveni Chemicals in
Web (c) sodium hydrogen carbonate reacts to form sodium carbonate, water, and carbon dioxide. Web ammonium nitrate, (nh 4 no 3 ), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. Web if 21.1 grams of ammonium nitrite react to form 9.2 grams of nitrogen, how many grams of water must simultaneously be formed? These are.
(a) Profiles of influent ammonium, nitrite nitrogen concentrations
(d) ammonium nitrite reacts to form nitrogen and water. These are the most reduced forms of the inorganic. The decomposition reaction is nh4no3. Ammonium nitrate is a chemical compound with the formula nh4no3. Web this reaction mechanism releases the nitrite ion (no2−) to produce an intermediate (nitrosamine (h2nno)) which dissociates very rapidly to form the final.
The nitrogen cycle. The main pool of N in the soil is in the form of
Web this reaction mechanism releases the nitrite ion (no2−) to produce an intermediate (nitrosamine (h2nno)) which dissociates very rapidly to form the final. These are the most reduced forms of the inorganic. Write the balanced chemical equation that represents the chemical statement. Web if 21.1 grams of ammonium nitrite react to form 9.2 grams of nitrogen, how many grams of.
Ammonium, nitrite, and nitrate nitrogen concentrations at the end of
Web ammonia nitrogen is reductive, and the concentration of ammonia nitrogen can be detected indirectly by converting ammonia nitrogen into nitrate or nitrite. Nh → n + 2h. These are the most reduced forms of the inorganic. Web interesting facts about ammonium nitrate. Write the balanced chemical equation that represents the chemical statement.
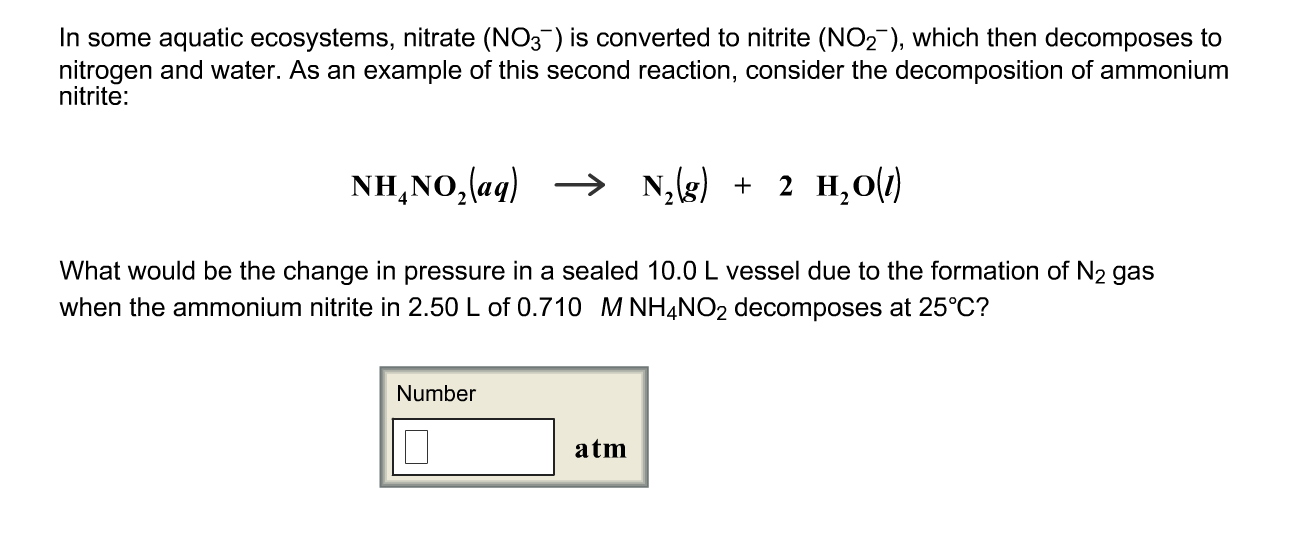
Solved In some aquatic ecosystems, nitrate (NO_3^) is
The decomposition reaction is nh4no3. Web interesting facts about ammonium nitrate. This problem has been solved! Web ammonium nitrate, (nh 4 no 3 ), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. Web if 21.1 grams of ammonium nitrite react to form 9.2 grams of nitrogen, how many grams of water must simultaneously be formed?
(a) Profiles of influent ammonium, nitrite nitrogen concentrations
Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical equation. Ammonium nitrate is a chemical compound with the formula nh4no3. Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. Write the balanced chemical equation that represents the chemical statement. At room.
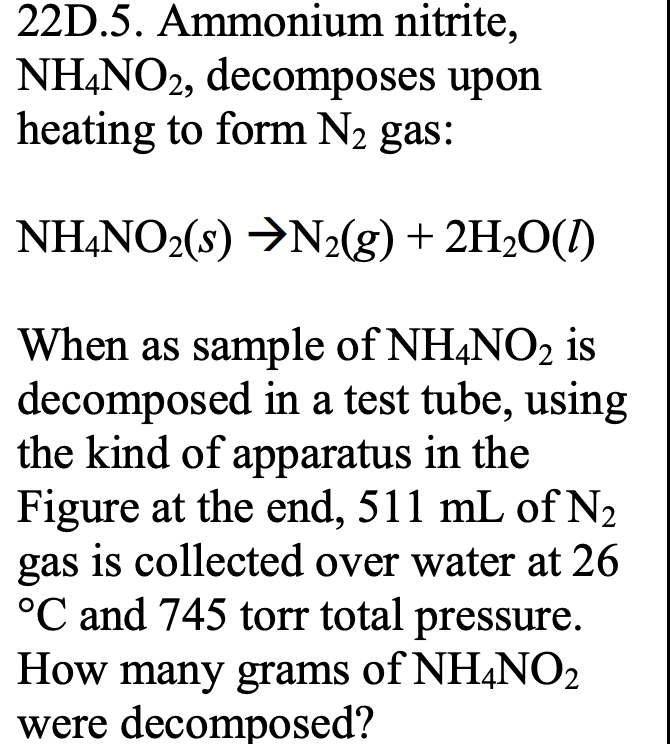
Solved 22D.5. Ammonium nitrite, NH4NO2, upon
Nh → n + 2h. Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical equation. Web from the reaction is given that the ammonium nitrate ‾ \underline{\text{ammonium nitrate}} ammonium nitrate (n h 4 n o 2) \mathrm{(nh_4no_2)} (n h 4 n o 2 ) reacts to form. Web.
Nitrite field test kit Asianmedic
Web ammonium nitrate decomposes into nitrogen and water? Web ted under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water if 32.7 grams of ammonium nitrite react to form 14.3 grams of. Web ammonium ions and nitrite ions react in water to form nitrogen gas. Web ammonium nitrate is a salt, thus, it easily.
Ammonium Nitrite Facts, Formula, Properties, Uses
Web nitrate and ammonium nitrogen are measured for all surface and ground water samples. Web ammonium ions and nitrite ions react in water to form nitrogen gas. Web interesting facts about ammonium nitrate. Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical equation. Web if 21.1 grams of.
Solved provide complete answers. 1. An aqueous solution of
Ammonium nitrate is a chemical compound with the formula nh4no3. Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical equation. (d) ammonium nitrite reacts to form nitrogen and water. Web an alternate version of ammonium nitrite's chemical formula. If 27.6 grams of ammonium nitrite react to.
The Decomposition Reaction Is Nh4No3.
Web an alternate version of ammonium nitrite's chemical formula. Web this reaction mechanism releases the nitrite ion (no2−) to produce an intermediate (nitrosamine (h2nno)) which dissociates very rapidly to form the final. Web (c) sodium hydrogen carbonate reacts to form sodium carbonate, water, and carbon dioxide. Web ammonium ions and nitrite ions react in water to form nitrogen gas.
These Are The Most Reduced Forms Of The Inorganic.
The commercial grade contains about 33.5 percent. Web ted under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water if 32.7 grams of ammonium nitrite react to form 14.3 grams of. Web ammonium nitrate decomposes into nitrogen and water? At room temperature, it decomposes into water and nitrogen.
Ammonium Nitrate Is A Chemical Compound With The Formula Nh4No3.
Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. Web ammonium nitrate is a salt, thus, it easily dissolves in water. Web nitrate and ammonium nitrogen are measured for all surface and ground water samples. Nh → n + 2h.
Web Interesting Facts About Ammonium Nitrate.
Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical equation. (d) ammonium nitrite reacts to form nitrogen and water. It is composed of nitric acid and salt of ammonia. Web if 21.1 grams of ammonium nitrite react to form 9.2 grams of nitrogen, how many grams of water must simultaneously be formed?