Ar Electron Configuration Long Form
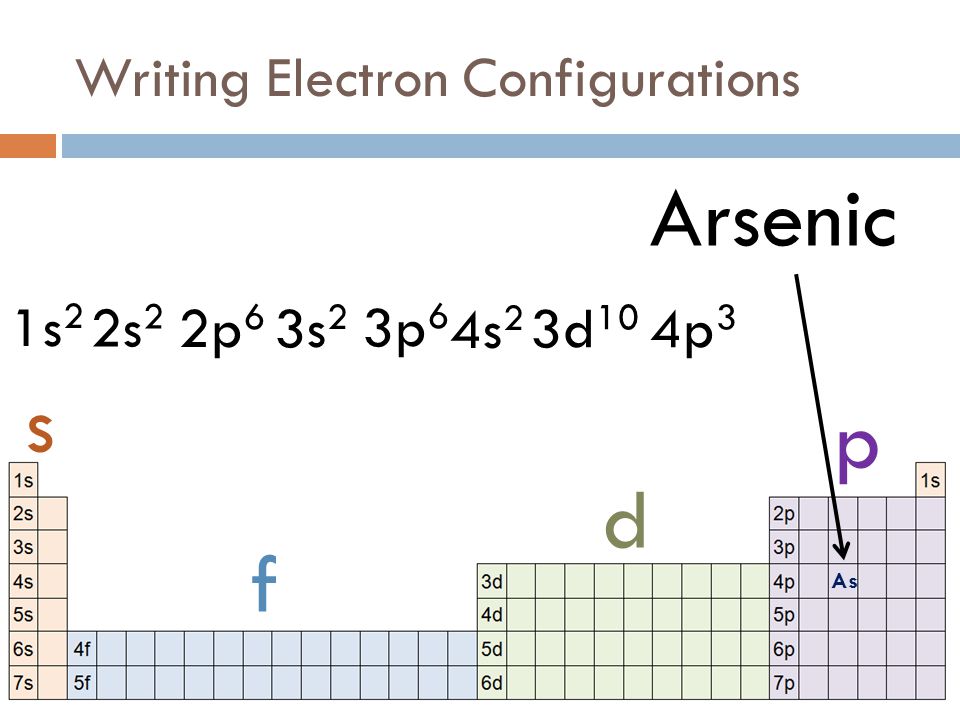
Ar Electron Configuration Long Form - For l orbit, n = 2. Long form (full configuration) short form (noble gas configuration) a) ar b) mg c) n d) li e) p f) cl. Fill in the orbital diagram. Web 2) write the electron configurations of the following elements: Argon has fully filled atomic orbital. Web write the electron configurations of each of these in long form and short form: Web the same rule will apply to transition metals when forming ions. As per the aufbau rule, the electrons will be filled. Web the electronic configuration of argon is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Web introduction before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations.
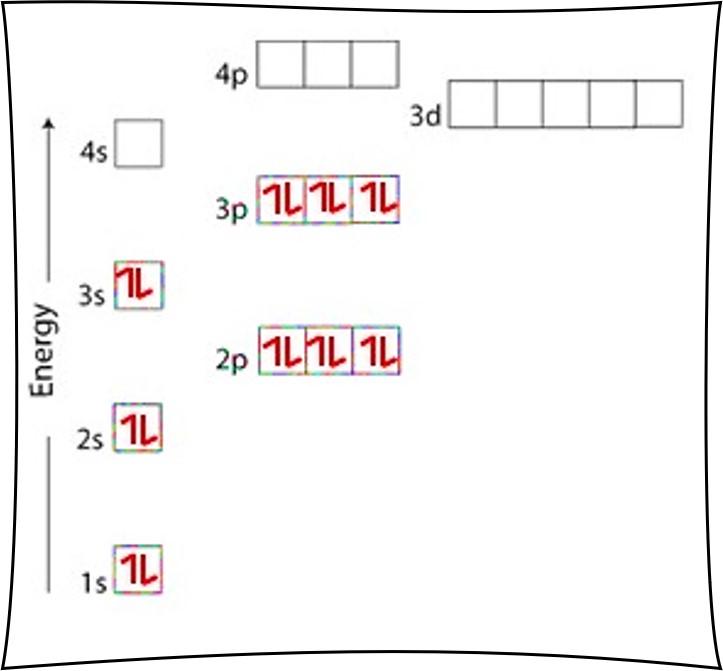
Web the first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). Web an argon atom is a neutral atom that has an atomic number of 18 which implies it has a total of 18 electrons. Web introduction before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Cl cl 1s 2s 2p 3s 3p 4s 4p 3d 7. Web the electronic configuration of argon is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. The maximum electron holding capacity in l orbit is 2n 2 = 2. Long form (full configuration) short form (noble gas configuration) a) ar b) mg c) n d) li e) p f) cl. Due to fully filled electronic configuration argon is least reactive. Web for example, n = 1 for k orbit. Web grayed out electron numbers indicate subshells filled to their maximum.
Web the electronic configuration of argon is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. The maximum electron holding capacity in l orbit is 2n 2 = 2. Argon has fully filled atomic orbital. Long form (full configuration) short form (noble gas configuration) a) ar b) mg c) n d) li e) p f) cl. The maximum electron holding capacity in k orbit is 2n 2 = 2 × 1 2 = 2. Bracketed noble gas symbols on the left represent inner configurations that are the same in each period. Web grayed out electron numbers indicate subshells filled to their maximum. The second electron also goes into the. We describe an electron configuration with a symbol that contains three. Web introduction before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations.
Electron Configuration Worksheet Easy Hard Science
Web write the electron configurations of each of these in long form and short form: Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The second electron also goes into the. We describe an electron configuration with a symbol that contains three. Web for example, n = 1 for k.
Electron Schematic Full Electron Configuration Of Argon
Argon has fully filled atomic orbital. Web the electronic configuration of argon is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Web an argon atom is a neutral atom that has an atomic number of 18 which implies it has a total of 18 electrons. We describe an electron configuration with a symbol.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio
Web the first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). Web grayed out electron numbers indicate subshells filled to their maximum. The second electron also goes into the. Web electron configuration the arrangements of electrons above the last (closed.
Scientific Explorer Atoms Part 4A Atoms and Chemistry Atomic
Web for example, n = 1 for k orbit. For l orbit, n = 2. Web the electronic configuration of argon is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. As per the aufbau rule, the electrons will be filled. Fill in the orbital diagram.
Argon, atomic structure Stock Image C018/3699 Science Photo Library
Web for example, n = 1 for k orbit. Web grayed out electron numbers indicate subshells filled to their maximum. Web the first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). Bracketed noble gas symbols on the left represent inner.
Provide the Electron Configuration for B Wardle Waycervours
Long form (full configuration) short form (noble gas configuration) a) ar b) mg c) n d) li e) p f) cl. Web the first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). For l orbit, n = 2. Web for.
Argon Electron Configuration YouTube
Web write the electron configurations of each of these in long form and short form: Web 2) write the electron configurations of the following elements: Web the same rule will apply to transition metals when forming ions. Web the electronic configuration of argon is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Due.
Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6
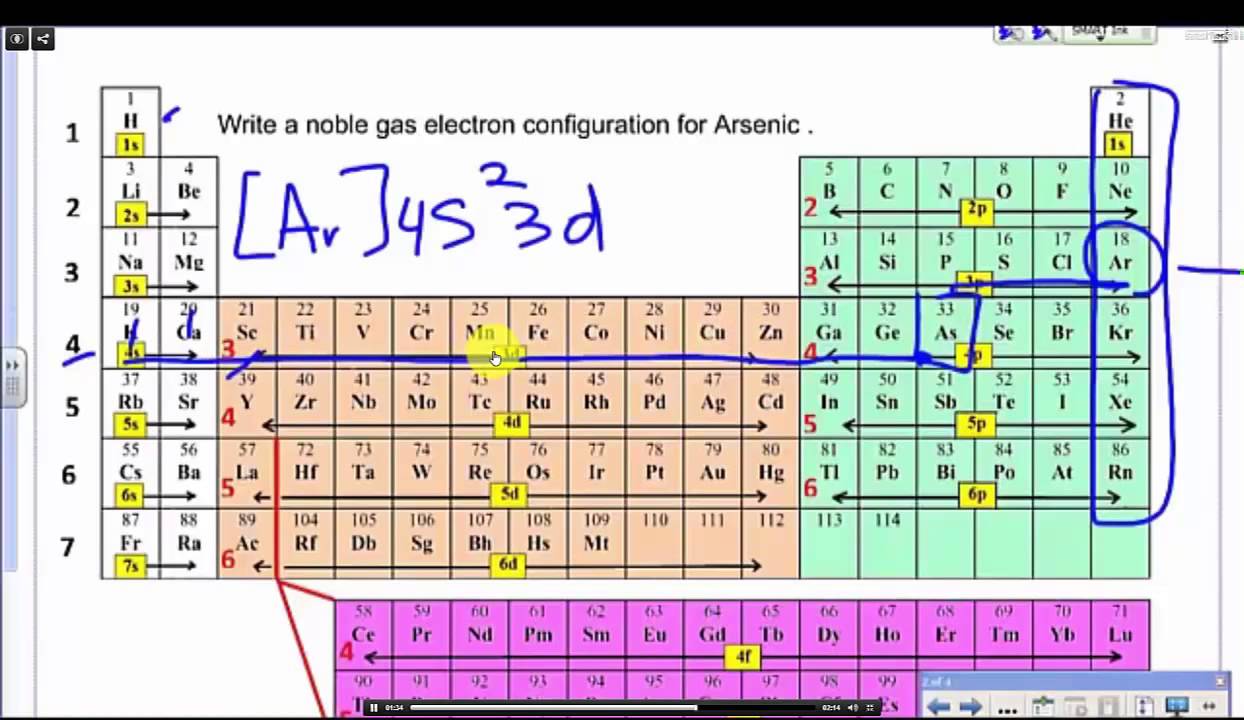
Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web 2) write the electron configurations of the following elements: Web the same rule will apply to transition metals when forming ions. The second electron also goes into the. As per the aufbau rule, the electrons will be filled.
Electronic Configuration of Argon (Ar)
For l orbit, n = 2. Web the first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). Web the electronic configuration of argon is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Bracketed.
Various Ways To Find A Electron Configuration for Argon
Web introduction before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Web for example, n = 1 for k orbit. Bracketed noble gas symbols on the left represent inner configurations that are the same in each period. Fill in the orbital diagram. The maximum electron holding capacity in l.
Web An Argon Atom Is A Neutral Atom That Has An Atomic Number Of 18 Which Implies It Has A Total Of 18 Electrons.
Web 2) write the electron configurations of the following elements: Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Web the first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). We describe an electron configuration with a symbol that contains three.
Argon Has Fully Filled Atomic Orbital.
Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web introduction before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. As per the aufbau rule, the electrons will be filled. Web write the electron configurations of each of these in long form and short form:
Web For Example, N = 1 For K Orbit.
The maximum electron holding capacity in k orbit is 2n 2 = 2 × 1 2 = 2. The second electron also goes into the. The maximum electron holding capacity in l orbit is 2n 2 = 2. Long form (full configuration) short form (noble gas configuration) a) ar b) mg c) n d) li e) p f) cl.
Cl Cl 1S 2S 2P 3S 3P 4S 4P 3D 7.
Web grayed out electron numbers indicate subshells filled to their maximum. For l orbit, n = 2. Due to fully filled electronic configuration argon is least reactive. Bracketed noble gas symbols on the left represent inner configurations that are the same in each period.