What Ion Does Fluorine Form
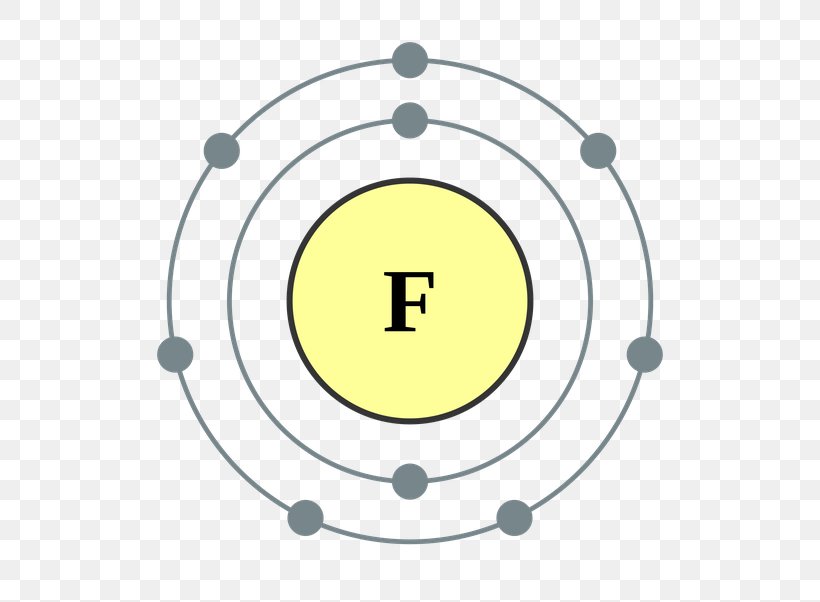
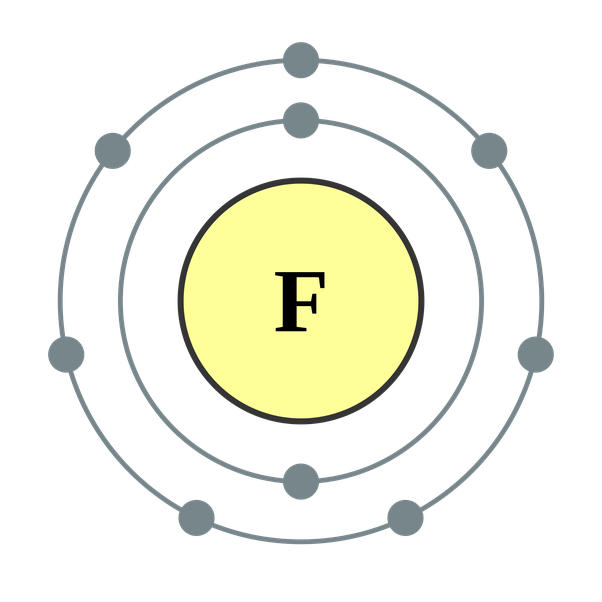
What Ion Does Fluorine Form - It has seven electrons in its outer shell. This is the same electron configuration as neon, which has atomic number 10, so it has 10 protons and 10. Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. Web does fluorine form a +1 ion? Web best answer copy there is only one possibility for the ion form of the element fluorine: Web what type of ion does fluorine form? Having a chemical formula of f−, fluoride ion is the simplest inorganic, monatomic anion of fluorine with basic properties. There are positive and negative ions. Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. It is the most electronegatve element.
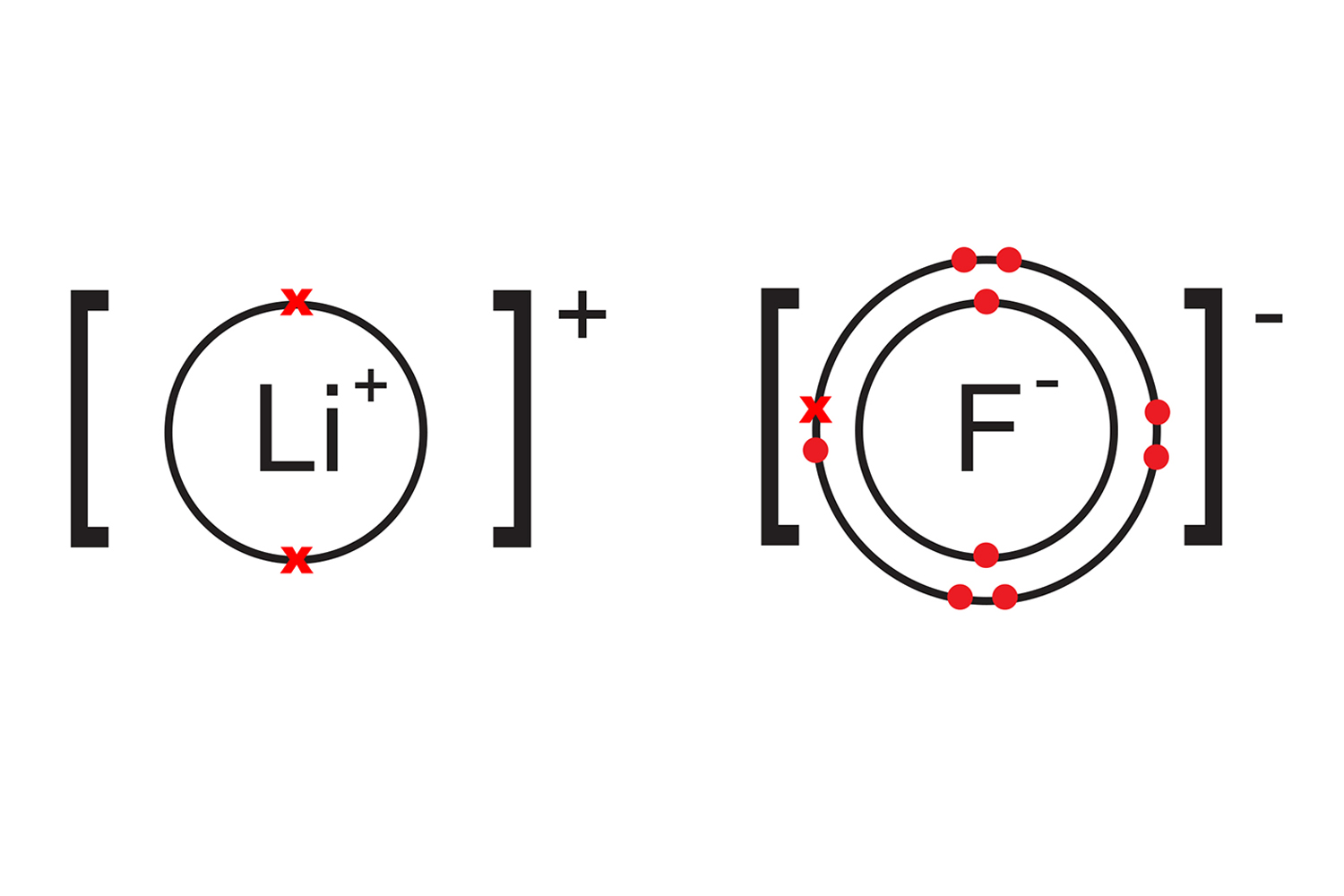
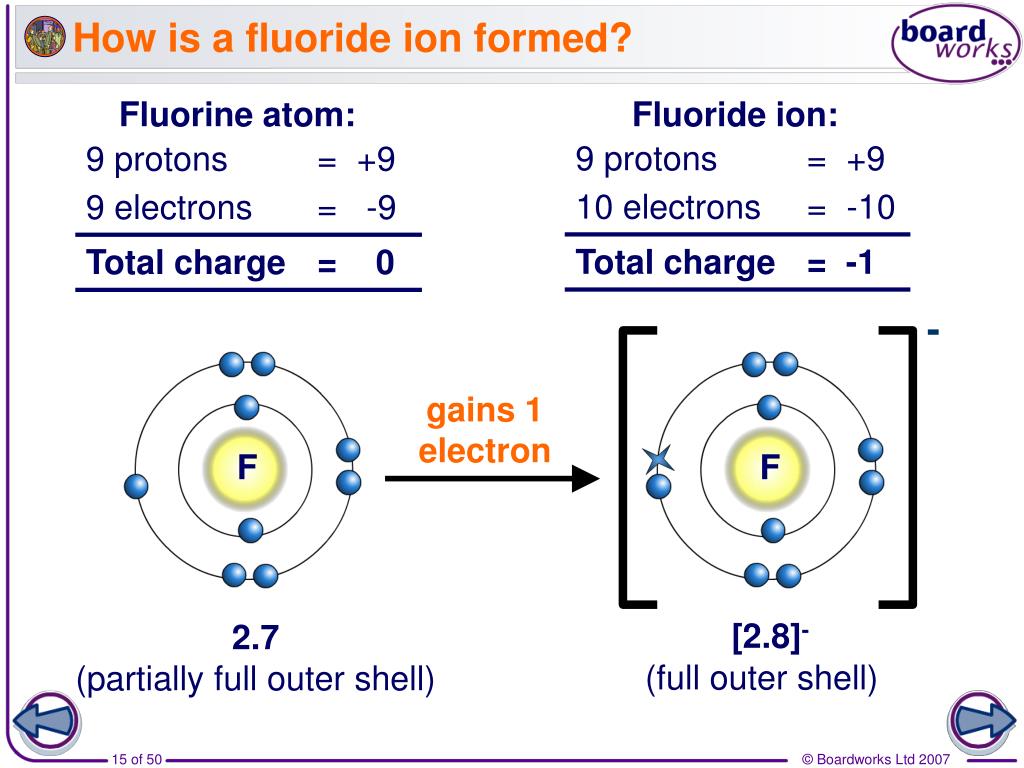
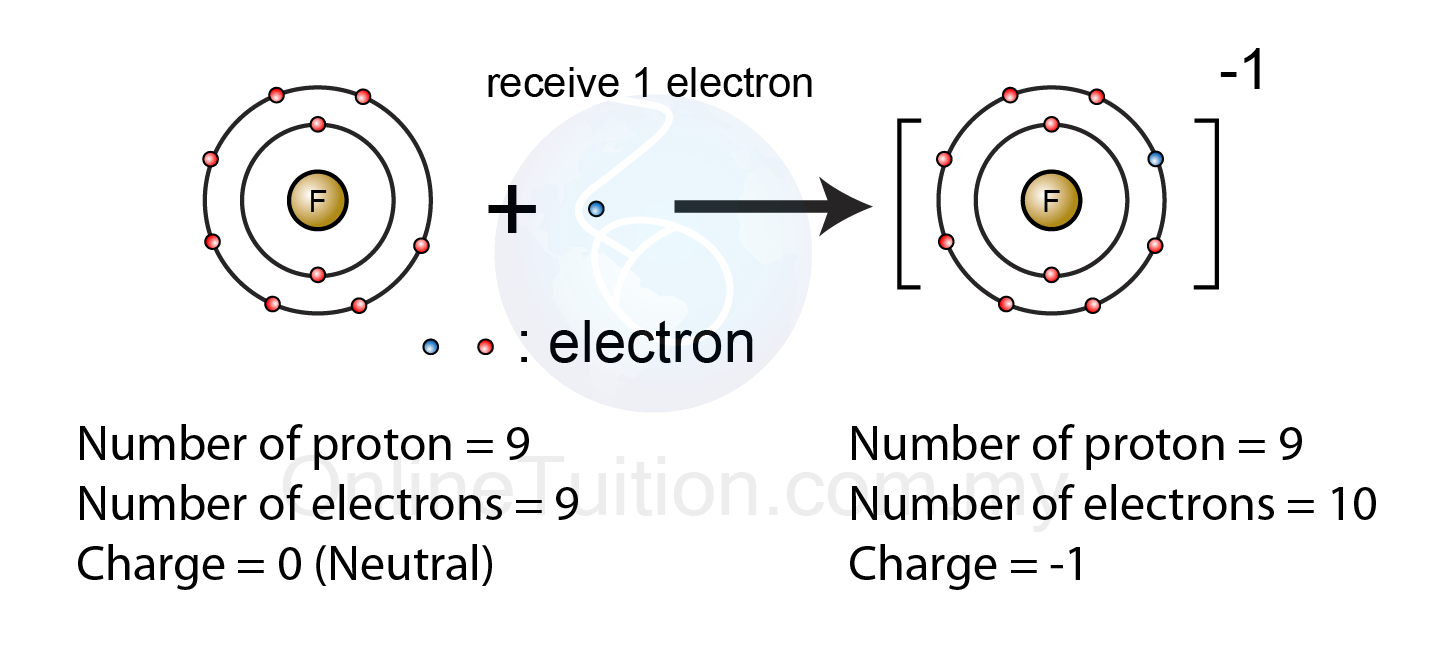
Web what is fluoride in chemistry? Web what type of ion does fluorine form? Having a chemical formula of f−, fluoride ion is the simplest inorganic, monatomic anion of fluorine with basic properties. Web the electron configuration of a f− ion is 1s22s22p6. Web does fluorine form a +1 ion? Web best answer copy there is only one possibility for the ion form of the element fluorine: A fluorine atom has nine protons and nine electrons, so it is. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two. Web an atom of fluorine in the gas phase, for example, gives off energy when it gains an electron to form an ion of fluorine. So a fluorine atom will take.
There are positive and negative ions. Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. [noun] a nonmetallic halogen element that is isolated as a pale yellowish flammable irritating toxic diatomic gas — see chemical elements table. Having a chemical formula of f−, fluoride ion is the simplest inorganic, monatomic anion of fluorine with basic properties. A fluorine atom has nine protons and nine electrons, so it is. Web the electron configuration of a f− ion is 1s22s22p6. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two. $$ 1s^22s^22p^5 $$ we see that $z=9$ and $s=2$, giving an effective nuclear charge of +7. It is the most electronegatve element. Web does fluorine form a +1 ion?
Gallery For > Pure Fluorine
Web taking fluorine as an example, the electron configuration is: So a fluorine atom will take. Web see answer (1) best answer copy anytime an atom gains an electron, it is gaining a negative charge. A fluorine atom has nine protons and nine electrons, so it is. This is the same electron configuration as neon, which has atomic number 10,.
Periodic Table Fluorine Valence Electrons Periodic Table Timeline
There are positive and negative ions. If atoms lose electrons, they become positive ions, or cations. A fluorine atom has nine protons and nine electrons, so it is. What is stable is a full outer shell of 8 electrons. Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,.
Valency is the number of bonds an atom can make with others
Web see answer (1) best answer copy anytime an atom gains an electron, it is gaining a negative charge. $$ 1s^22s^22p^5 $$ we see that $z=9$ and $s=2$, giving an effective nuclear charge of +7. If atoms lose electrons, they become positive ions, or cations. [noun] a nonmetallic halogen element that is isolated as a pale yellowish flammable irritating toxic.
Fluorine by Kade Moural
Web the electron configuration of a f− ion is 1s22s22p6. Web what type of ion does fluorine form? Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. Having a chemical formula of f−, fluoride ion is the simplest inorganic, monatomic anion of fluorine with basic properties. There are positive and negative ions.
How to Find the Ionic Charge for Fluorine (F) YouTube
$$ 1s^22s^22p^5 $$ we see that $z=9$ and $s=2$, giving an effective nuclear charge of +7. Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. Web the electron configuration of a f− ion is 1s22s22p6. Web does fluorine form a +1 ion? It has seven electrons in its outer shell.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web the electron configuration of a f− ion is 1s22s22p6. Web what type of ion does fluorine form? Web best answer copy there is only one possibility for the ion form of the element fluorine: Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. Web an atom of fluorine in the gas phase, for example,.
Formation of Negative Ions SPM Chemistry
If atoms lose electrons, they become positive ions, or cations. Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. Web what type of ion does fluorine form? It is the most electronegatve element. It has seven electrons in its outer shell.
PPT Stability and Ionic Bonding PowerPoint Presentation ID1443529
Web what type of ion does fluorine form? Web the electron configuration of a f− ion is 1s22s22p6. Web see answer (1) best answer copy anytime an atom gains an electron, it is gaining a negative charge. Web to 31 f (with the exception of 30 f ) and two isomers ( 18m f and 26m f ). So a.
Where are valence electrons found? + Example
Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,. There are positive and negative ions. It is the most electronegatve element. Web what type of ion does fluorine form? Web best answer copy there is only one possibility for the ion form of the element fluorine:
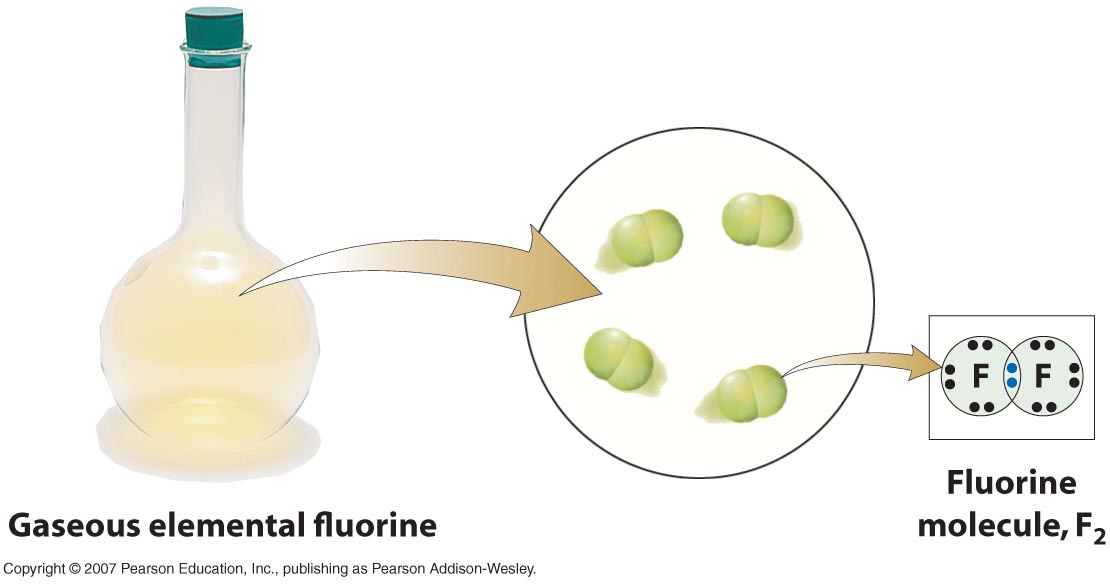
Why do two fluorine atoms bond together? Socratic
Web to 31 f (with the exception of 30 f ) and two isomers ( 18m f and 26m f ). Web taking fluorine as an example, the electron configuration is: A fluorine atom has nine protons and nine electrons, so it is. Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. What is stable is.
Web See Answer (1) Best Answer Copy Anytime An Atom Gains An Electron, It Is Gaining A Negative Charge.
Web taking fluorine as an example, the electron configuration is: [noun] a nonmetallic halogen element that is isolated as a pale yellowish flammable irritating toxic diatomic gas — see chemical elements table. Web an atom of fluorine in the gas phase, for example, gives off energy when it gains an electron to form an ion of fluorine. Web does fluorine form a +1 ion?
It Has Seven Electrons In Its Outer Shell.
Web best answer copy there is only one possibility for the ion form of the element fluorine: Web what type of ion does fluorine form? If atoms lose electrons, they become positive ions, or cations. Web the electron configuration of a f− ion is 1s22s22p6.
$$ 1S^22S^22P^5 $$ We See That $Z=9$ And $S=2$, Giving An Effective Nuclear Charge Of +7.
Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two. A fluorine atom has nine protons and nine electrons, so it is. What is stable is a full outer shell of 8 electrons. Since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e.,.
Web To 31 F (With The Exception Of 30 F ) And Two Isomers ( 18M F And 26M F ).
Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. It is the most electronegatve element. Having a chemical formula of f−, fluoride ion is the simplest inorganic, monatomic anion of fluorine with basic properties. So a fluorine atom will take.