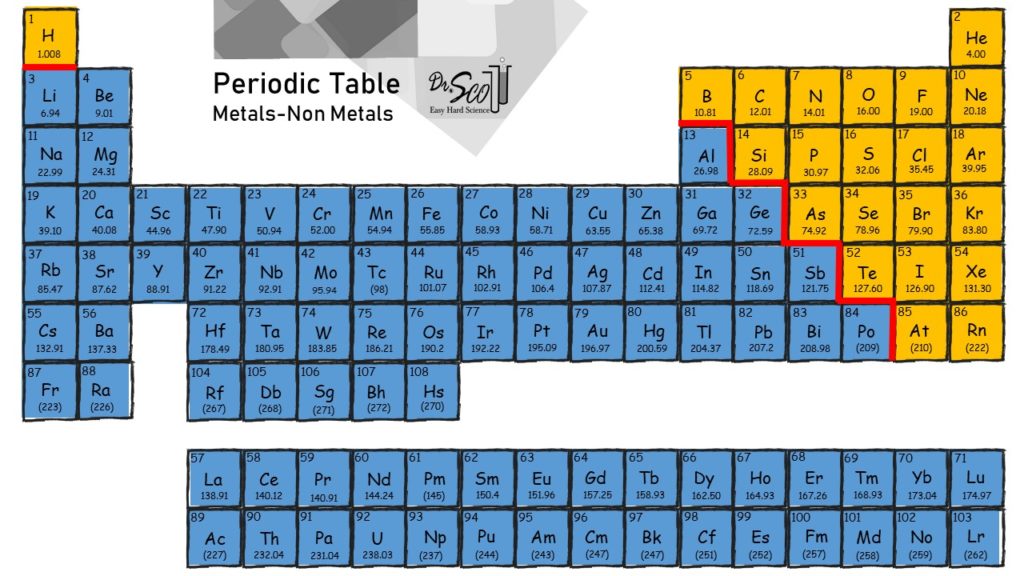
What Kinds Of Elements Form Ionic Bonds
What Kinds Of Elements Form Ionic Bonds - An atom of chlorine will gain. An atom of sodium will lose an electron and form a positive ion. Web ionic and covalent bonding. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. 2) another example, magnesium and oxygen. Web which elements form ionic bonds? 1) for example, consider na and cl. This exchange results in a more stable, noble gas. Web answer (1 of 3): The alkali halides (nacl, lif, etc.).
There are primarily two forms of bonding that an atom can participate in: The chlorine atom has seven electrons in its outer shell. The alkali halides (nacl, lif, etc.). 2) another example, magnesium and oxygen. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. Charged chemical species form when neutral atoms, or groups of atoms, lose. An atom of sodium will lose an electron and form a positive ion. Boiling and melting point 4. Web it is now called a sodium ion. Web what type of elements form ionic bonds?
Web answer (1 of 3): An atom of chlorine will gain. Web which elements form ionic bonds? The alkali halides (nacl, lif, etc.). This exchange results in a more stable, noble gas. Web it is now called a sodium ion. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Web ionic bonds are one of the two main types of chemical bonds. Web what type of elements form ionic bonds?
2.6 Molecules and Molecular Compounds Chemistry LibreTexts
Web ionic and covalent bonding. Charged chemical species form when neutral atoms, or groups of atoms, lose. Web what type of elements form ionic bonds? There are primarily two forms of bonding that an atom can participate in: 3) last example, mg and cl.
Ionic Properties
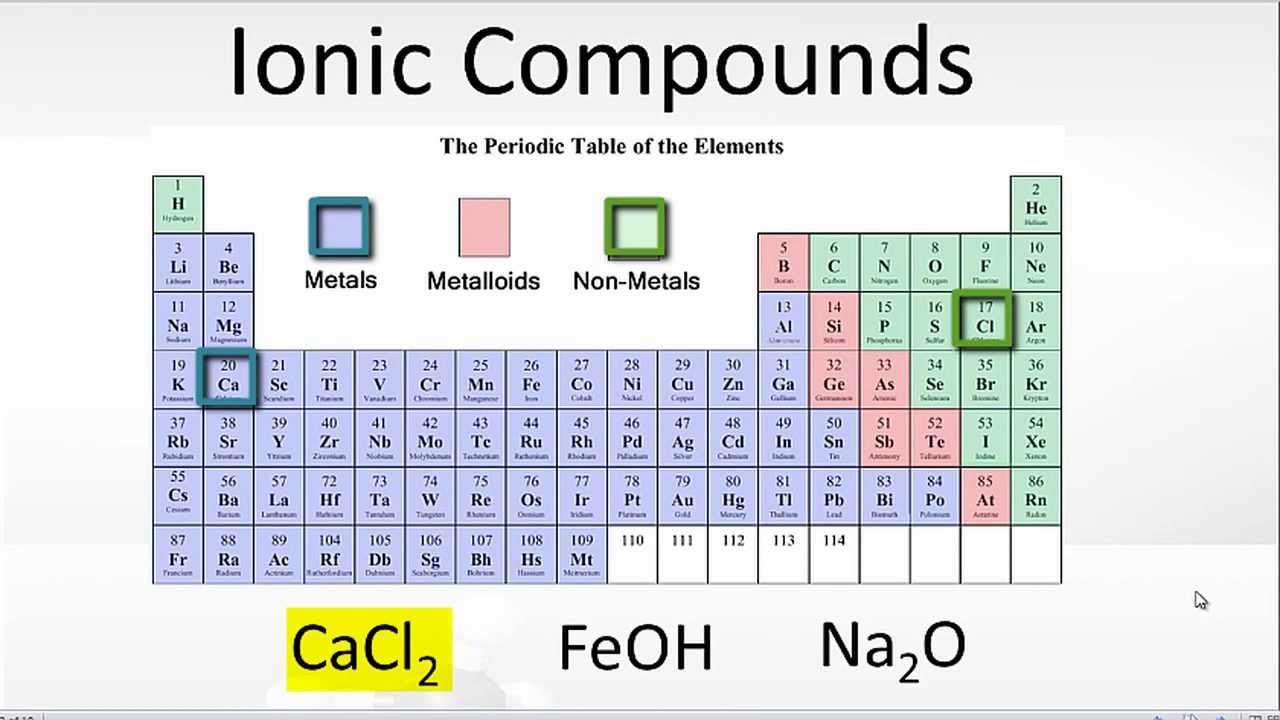
Boiling and melting point 4. The alkali halides (nacl, lif, etc.). 2) another example, magnesium and oxygen. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals).
Naming Simple Ionic Compounds Pathways to Chemistry
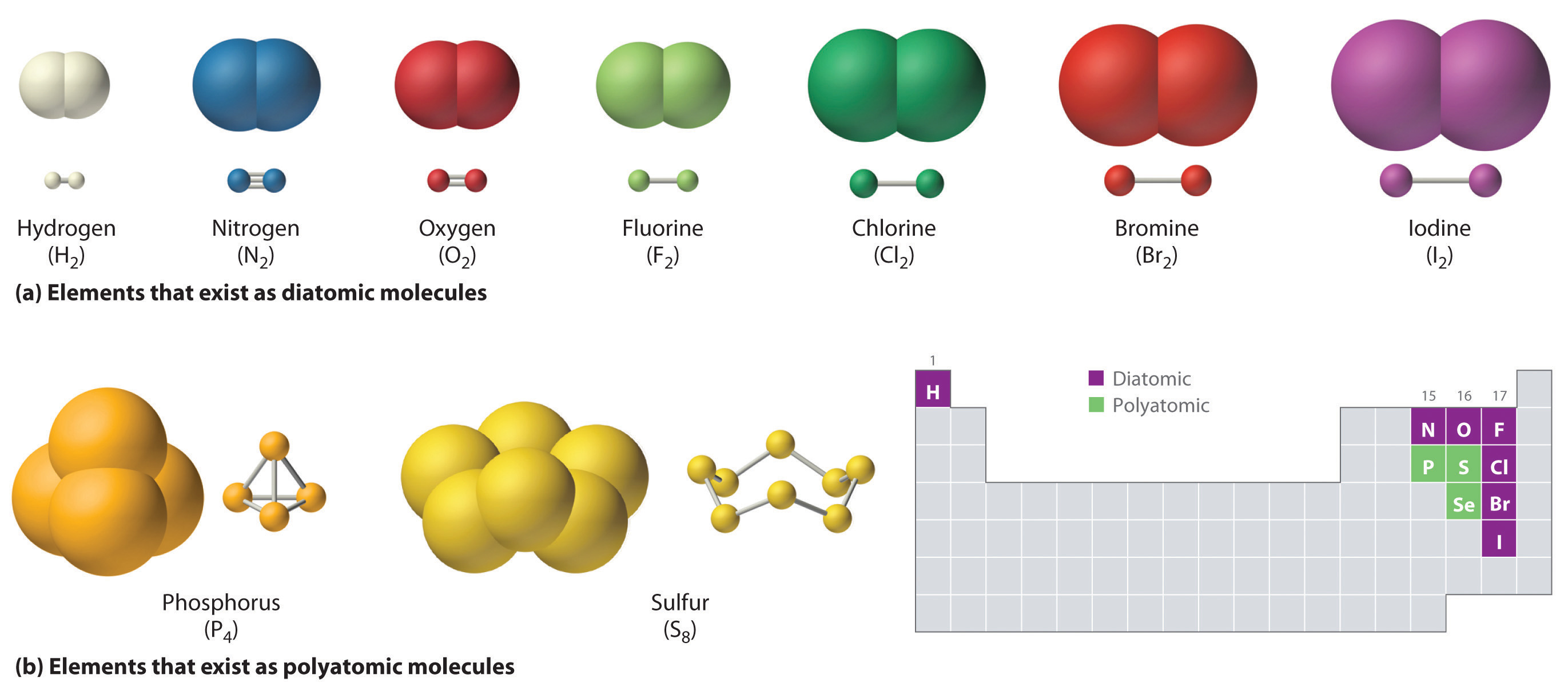
Web ionic and covalent bonding. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. 1) for example, consider na and cl. Covalent bonding involves the sharing of electrons. Web ionic bonds are one of the two main types of chemical bonds.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Charged chemical species form when neutral atoms, or groups of atoms, lose. The alkali halides (nacl, lif, etc.). 3) last example, mg and cl. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). The chlorine atom has seven electrons in its outer shell.
Examples of Ionic Bonding YouTube
Electrical conductivity how are ionic bonds formed? Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Boiling and melting point 4. Charged chemical species form when neutral atoms, or groups of atoms, lose. Web it is now called a sodium ion.
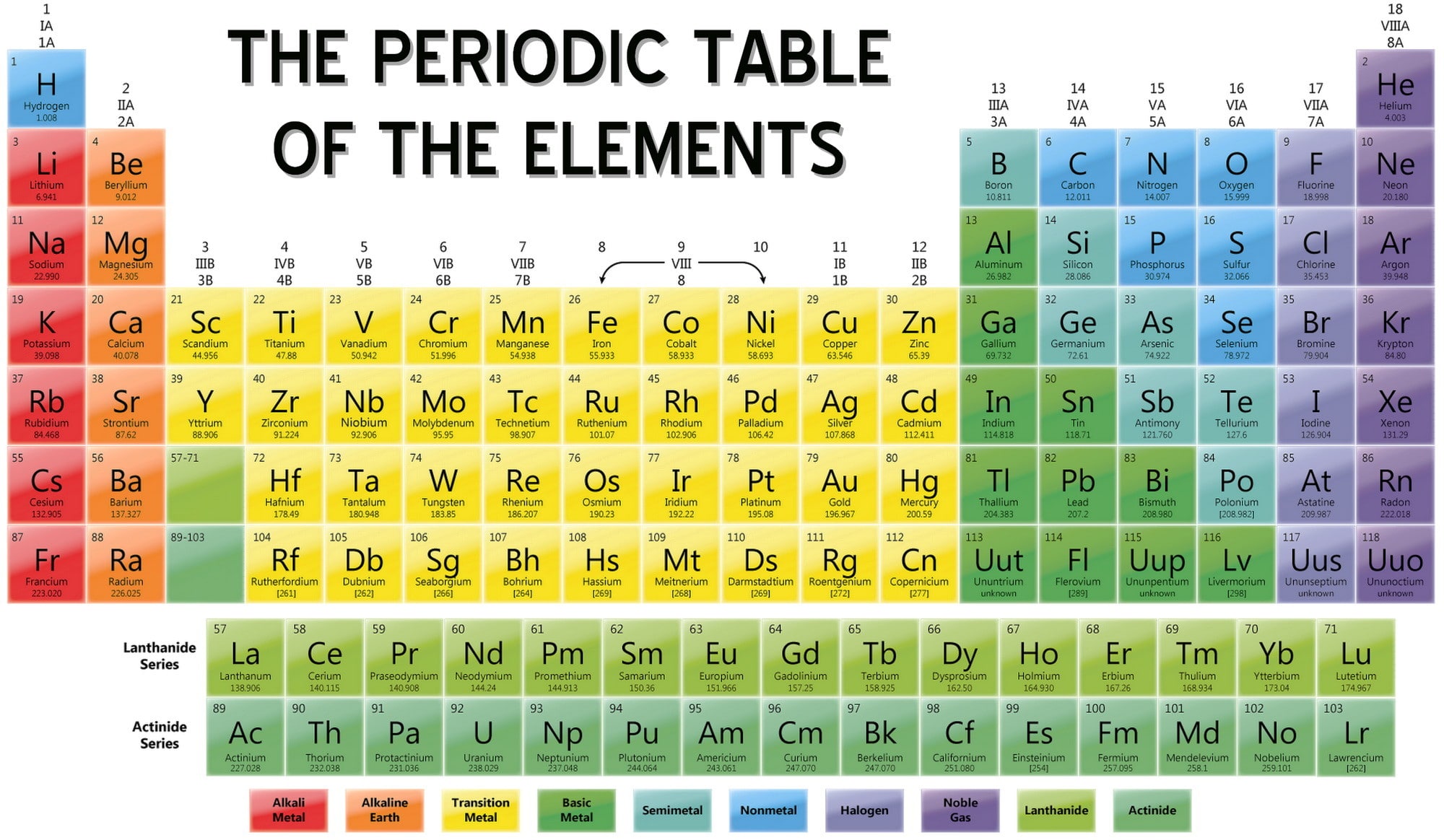
Periodic Table of Elements & Ionic Bonding Mrs. Zeringue's 7th Grade
Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). 3) last example, mg and cl. Web answer (1 of 3): This exchange results in a more stable, noble gas. Boiling and melting point 4.
Examples of Ionic Bonds and Compounds
An atom of chlorine will gain. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. 4/28/2022 wiki user ∙ 14y ago study now see answers (3) best answer copy metals form ionic bonds with. Web which elements form ionic bonds? Electrical conductivity how are ionic bonds formed?
Ionic Bond Definition Easy Hard Science
Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. 2) another example, magnesium and oxygen. Web which elements form ionic bonds? 1) for example, consider na and cl. Web it is now called a sodium ion.
10 Notable Differences Between Ionic And Covalent Bonds Current
Covalent bonding involves the sharing of electrons. Web it is now called a sodium ion. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Web ionic and covalent bonding. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared.
Ionic Bond Definition, Types, Properties & Examples
Web ionic and covalent bonding. Charged chemical species form when neutral atoms, or groups of atoms, lose. Web answer (1 of 3): An atom of sodium will lose an electron and form a positive ion. The chlorine atom has seven electrons in its outer shell.
Ionic Bonding Is A Type Of Chemical Bond In Which Valence Electrons Are Lost From One Atom And Gained By Another.
Web which elements form ionic bonds? Covalent bonding involves the sharing of electrons. 4/28/2022 wiki user ∙ 14y ago study now see answers (3) best answer copy metals form ionic bonds with. Charged chemical species form when neutral atoms, or groups of atoms, lose.
An Atom Of Chlorine Will Gain.
Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. 1) for example, consider na and cl. The chlorine atom has seven electrons in its outer shell. An atom of sodium will lose an electron and form a positive ion.
The Alkali Halides (Nacl, Lif, Etc.).
3) last example, mg and cl. There are primarily two forms of bonding that an atom can participate in: Web it is now called a sodium ion. Web what type of elements form ionic bonds?
Web Ionic Bonds Are One Of The Two Main Types Of Chemical Bonds.
Web answer (1 of 3): Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. Web ionic and covalent bonding. This exchange results in a more stable, noble gas.



/ionic-bond-58fd4ea73df78ca1590682ad.jpg)