When Group 2A Elements Form Ions They
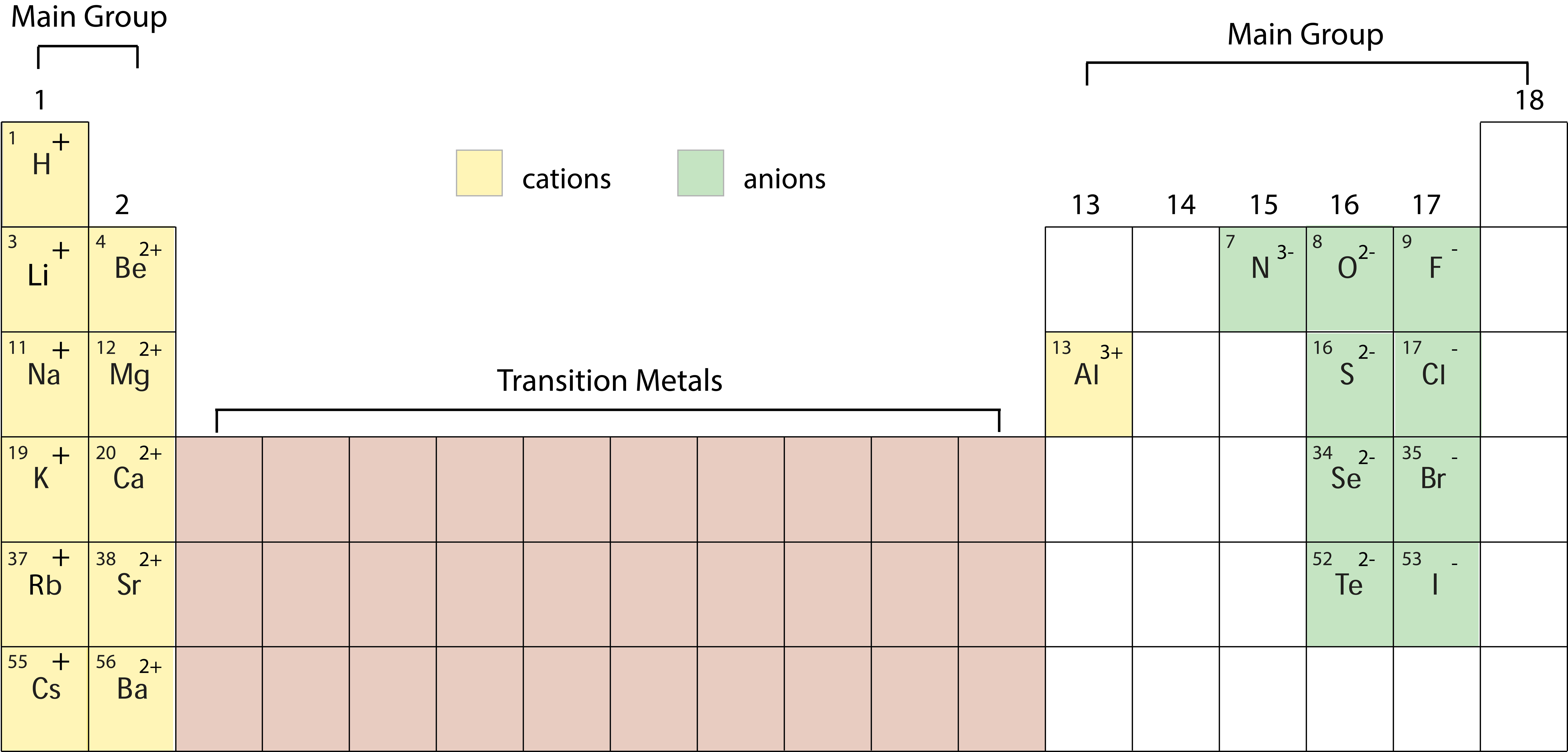
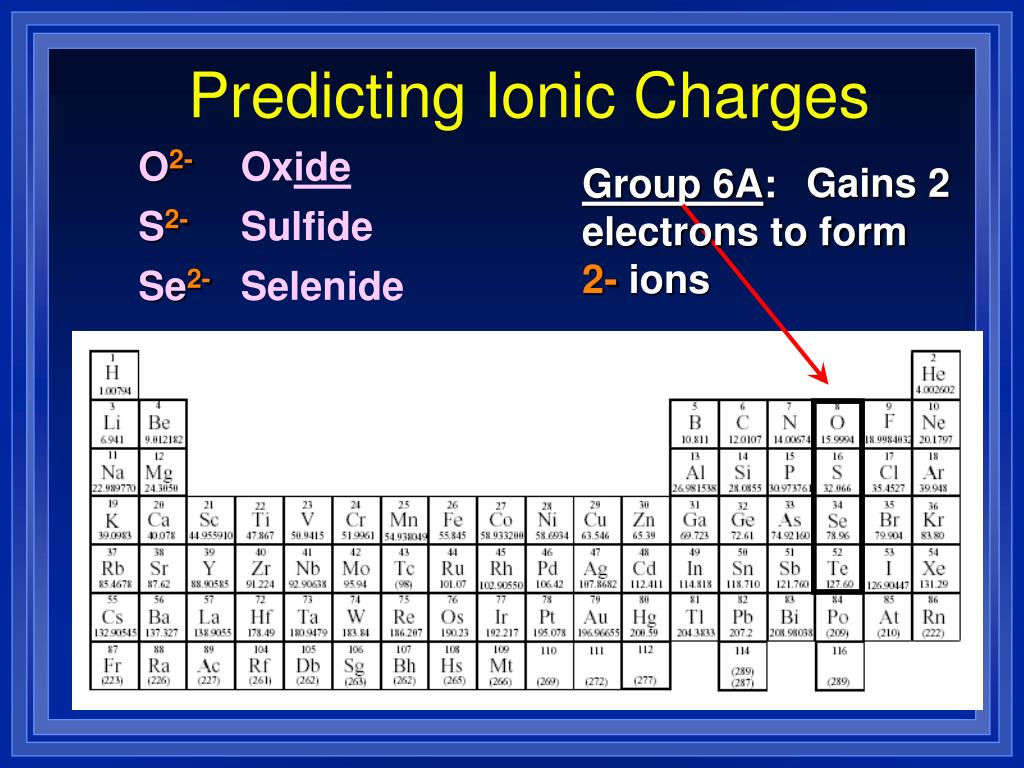
When Group 2A Elements Form Ions They - When group 2a elements form ions, they. Metals are electron rich substances. The number of valence electrons an atom has will. Metals are electron rich substances. Web answer they lose 2 electrons. When naming a transition metal ion that can have more. Using periodic properties to identify group 2a cations and group 7a anions suppose that substances \(\ce{x}\), \(\ce{y}\), and. It does not change its number of electrons. As you know, neutral atoms become ions by losing or by gaining electrons. M g → m g2+ + 2e−.
Lose two electrons aluminum is a group 3a metal. They tend to lose electrons to form positive ions. Metals are electron rich substances. When atom lose electron positive charge is created on atom. A sodium atom loses one electron to form a sodium ion forming negative ions 1, 2 and 3, the number of electrons lost is the same as the group number. Web group 2 elements are alkaline earth metals in which beryllium, magnesium, calcium, strontium, barium and radium are present when group 2 elements form ions. Roman numeral following the name when naming a transition metal ion that can. Web when group 2a elements form ions, they. They tend to lose electrons to form positive ions.
It does not change its number of electrons. Web when group 2a elements form ions what happens? When an atom loses are gain the electrons ions are formed. A sodium atom loses one electron to form a sodium ion forming negative ions M g → m g2+ + 2e−. Web group 2 elements are alkaline earth metals in which beryllium, magnesium, calcium, strontium, barium and radium are present when group 2 elements form ions. Web when group 2a elements form ions, they. X → x⁺ + e⁻. Lose two electrons aluminum is a group 3a metal. They tend to lose electrons to form positive ions.
Chem Ions Scientific Tutor
They tend to lose electrons to form positive ions. When naming a transition metal ion that can have more. A sodium atom loses one electron to form a sodium ion forming negative ions 1, 2 and 3, the number of electrons lost is the same as the group number. Beryllium (be), magnesium (mg), calcium (ca), strontium (sr), barium (ba), and.
The Periodic Table and its Design Pathways to Chemistry
When group 2a elements form ions, they. Web group 2 elements are alkaline earth metals in which beryllium, magnesium, calcium, strontium, barium and radium are present when group 2 elements form ions. When naming a transition metal ion that can have more. A sodium atom loses one electron to form a sodium ion forming negative ions Web when group 2a.
table of elements chart
Web test match created by dkmoran terms in this set (47) horizontal row in the periodic table period vertical column in the periodic table group basis for mendeleev's arrangement of. When naming a transition metal ion that can have more. Web answer they lose 2 electrons. They tend to lose electrons to form positive ions. It is formed when an.
Chemistry Chp 9 Chemical Names and Formulas PowerPoint
It does not change its number of electrons. Web in any chemical compound, the masses of elements are always in the same proportion by mass. Lose two electrons aluminum is a group 3a metal. A sodium atom loses one electron to form a sodium ion forming negative ions When group 2a elements form ions, they lose two electrons.
CH103 CHAPTER 4 Ions and Ionic Compounds Chemistry
Web group 2 elements are alkaline earth metals in which beryllium, magnesium, calcium, strontium, barium and radium are present when group 2 elements form ions. Calcium does not obey the octet rule. There are two types of ions. Web when group 2a elements form ions what happens? Anion cation 1 = anion it is.
Pin on Education
Using periodic properties to identify group 2a cations and group 7a anions suppose that substances \(\ce{x}\), \(\ce{y}\), and. Web group 2 elements are alkaline earth metals in which beryllium, magnesium, calcium, strontium, barium and radium are present when group 2 elements form ions. A sodium atom loses one electron to form a sodium ion forming negative ions When group 2a.
PreChemistry
Web test match created by dkmoran terms in this set (47) horizontal row in the periodic table period vertical column in the periodic table group basis for mendeleev's arrangement of. M g → m g2+ + 2e−. Web answer they lose 2 electrons. When atom lose electron positive charge is created on atom. They tend to lose electrons to form.
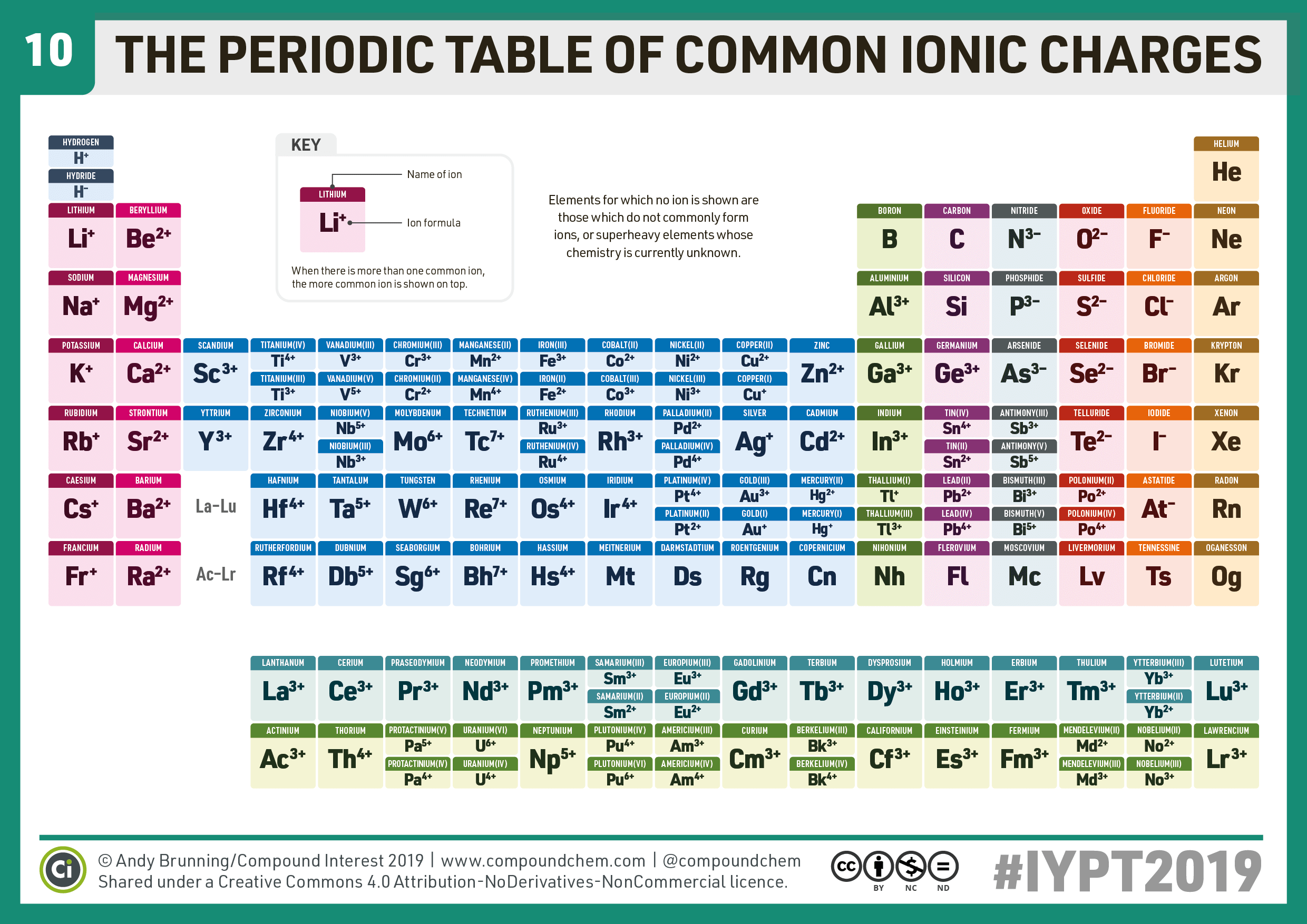
10 Periodic Table of Common Ions Compound Interest
A sodium atom loses one electron to form a sodium ion forming negative ions They tend to lose electrons to form positive ions. It is formed when an atom loses the electrons. Metals are electron rich substances. Web answer they lose 2 electrons.
Chemistry Chp 9 Chemical Names and Formulas PowerPoint
X → x⁺ + e⁻. When group 2a elements form ions, they lose two electrons. Web in any chemical compound, the masses of elements are always in the same proportion by mass. When an atom loses are gain the electrons ions are formed. Web group 2a (or iia) of the periodic table are the alkaline earth metals :
PPT Chapter 9 “Chemical Names and Formulas” PowerPoint Presentation
Using periodic properties to identify group 2a cations and group 7a anions suppose that substances \(\ce{x}\), \(\ce{y}\), and. Metals are electron rich substances. Web answer they lose 2 electrons. A sodium atom loses one electron to form a sodium ion forming negative ions They tend to lose electrons to form positive ions.
When Two Elements Form More Than One Compound,.
They tend to lose electrons to form positive ions. There are two types of ions. Beryllium (be), magnesium (mg), calcium (ca), strontium (sr), barium (ba), and radium (ra). Web in any chemical compound, the masses of elements are always in the same proportion by mass.
When Group 2A Elements Form Ions, They.
Roman numeral following the name when naming a transition metal ion that can. A sodium atom loses one electron to form a sodium ion forming negative ions It is formed when an atom loses the electrons. Web when group 2a elements form ions what happens?
As You Know, Neutral Atoms Become Ions By Losing Or By Gaining Electrons.
Metals are electron rich substances. Web for elements in groups. When atom lose electron positive charge is created on atom. M g → m g2+ + 2e−.
Anion Cation 1 = Anion It Is.
1, 2 and 3, the number of electrons lost is the same as the group number. Calcium does not obey the octet rule. Web when group 2a elements form ions, they. Web group 2 elements are alkaline earth metals in which beryllium, magnesium, calcium, strontium, barium and radium are present when group 2 elements form ions.