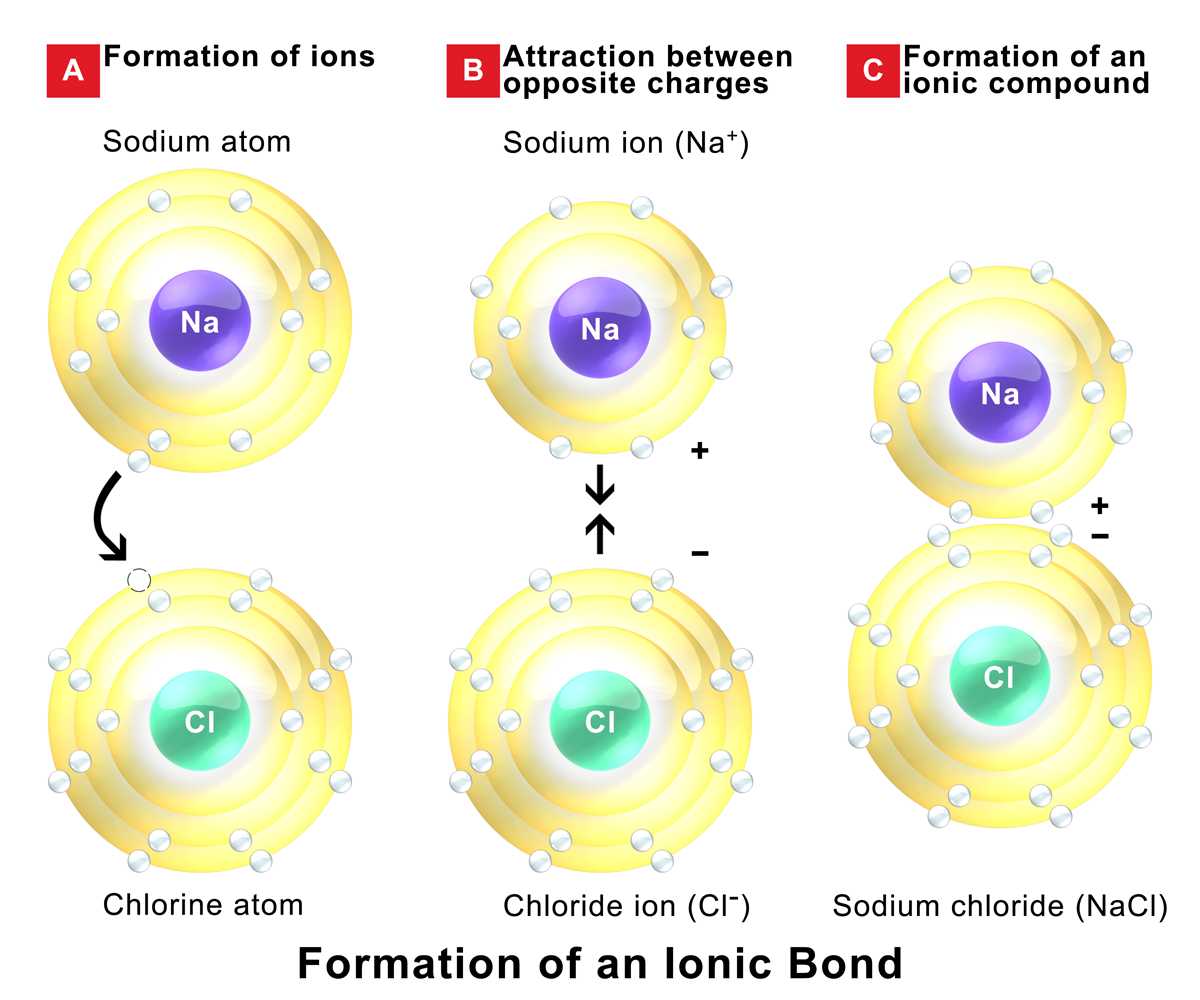
Why Do Ionic Bonds Form Between Metals And Nonmetals
Why Do Ionic Bonds Form Between Metals And Nonmetals - That’s because metals want to give up electrons, and nonmetals want to gain electrons. Ionic bonds are the strongest type of bond in the world and. An ionic bond is a type of. Ions with opposite charges will attract one another creating an ionic bond. Web part of chemistry (single science) chemical patterns revise test 1 2 3 4 5 6 7 forming ionic compounds metalatoms have only a few electrons in their outer shell whereas non. Are oxidized) when they undergo chemical reactions they. Web ionic bonds occur between metals, losing electrons, and nonmetals, gaining electrons. Web in order to become stable, metal atoms lose electrons. Metals tend to have low ionization energies, and typically lose electrons (i.e. Web the interaction of metals and halogens results in the creation of ionic bonds.
Web ionic bonds occur between anions and cations. Metals tend to have low ionization energies, and typically lose electrons (i.e. Web part of chemistry (single science) chemical patterns revise test 1 2 3 4 5 6 7 forming ionic compounds metalatoms have only a few electrons in their outer shell whereas non. Web ionic bonds form only between metals and nonmetals. Web the interaction of metals and halogens results in the creation of ionic bonds. An ionic bond is a type of. Are oxidized) when they undergo chemical reactions they. Web as all ionic compounds are polar they form salts. That’s because metals “want” to give up electrons, and nonmetals “want” to gain electrons. Chemistry bonding basics ionic bonding 1 answer anor277 feb 13, 2017 they can.
Web part of chemistry (single science) chemical patterns revise test 1 2 3 4 5 6 7 forming ionic compounds metalatoms have only a few electrons in their outer shell whereas non. Ionic bonds form only between metals and nonmetals. Web the interaction of metals and halogens results in the creation of ionic bonds. That’s because metals “want” to give up electrons, and nonmetals “want” to gain electrons. Web compounds between metals and nonmetals are predominantly ionic because there is a large difference in electronegativity between most metals and most. Ionic bonds are the strongest type of bond in the world and. Web ionic bonds occur between anions and cations. Anions are ions that have negative. An ionic bond is a type of. Web when ionic bonds form, a metal donates one or more electrons, due to having a low electronegativity, to form a positive ion or cation.
Ionic Bonding BOOM Cards Ionic bonding, Science teaching resources
Web nonmetals lose electrons to gain negative charge and form anions, whereas metals lose electrons to gain positive charge and form cations. Chemistry bonding basics ionic bonding 1 answer anor277 feb 13, 2017 they can. Web ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Ionic bonds.
Ionic Properties
Web answer (1 of 6): Salts(ionic compounds) are generally crystalline in nature and hence contain water of crystallization. Web nonmetals lose electrons to gain negative charge and form anions, whereas metals lose electrons to gain positive charge and form cations. Ionic bonds are the strongest type of bond in the world and. Ionic bonds are chemical bonds formed by the.
10 Notable Differences Between Ionic And Covalent Bonds Current
Ionic bonds are the strongest type of bond in the world and. Web the interaction of metals and halogens results in the creation of ionic bonds. Ions with opposite charges will attract one another creating an ionic bond. Ionic bonds form only between metals and nonmetals. Are oxidized) when they undergo chemical reactions they.
Examples of Ionic Bonds and Ionic Compounds
Salts(ionic compounds) are generally crystalline in nature and hence contain water of crystallization. Web in order to become stable, metal atoms lose electrons. Web ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Web the interaction of metals and halogens results in the creation of ionic bonds..
Ionic Bond Definition, Types, Properties & Examples
Web when ionic bonds form, a metal donates one or more electrons, due to having a low electronegativity, to form a positive ion or cation. Salts(ionic compounds) are generally crystalline in nature and hence contain water of crystallization. Web as all ionic compounds are polar they form salts. That’s because metals “want” to give up electrons, and nonmetals “want” to.
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
Are oxidized) when they undergo chemical reactions they. Ionic bonds form only between metals and nonmetals. Anions are ions that have negative. Web part of chemistry (single science) chemical patterns revise test 1 2 3 4 5 6 7 forming ionic compounds metalatoms have only a few electrons in their outer shell whereas non. That’s because metals “want” to give.
Student Exploration Ionic Bonds Answer Key Quizlet / Ionic Bonds Gizmo
Web ionic bonds form only between metals and nonmetals. Anions are ions that have negative. Web ionic bonds form only between metals and nonmetals. Web ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Ionic bonds form only between metals and nonmetals.
Ionic Bonds in 2020 Ionic bonding, Chemical bond, Ionic
Ionic bonds are the strongest type of bond in the world and. Ions with opposite charges will attract one another creating an ionic bond. Chemistry bonding basics ionic bonding 1 answer anor277 feb 13, 2017 they can. Web in order to become stable, metal atoms lose electrons. Web ionic bonds occur between metals, losing electrons, and nonmetals, gaining electrons.
Ionic Bond Definition Easy Hard Science
That’s because metals want to give up electrons, and nonmetals want to gain electrons. Web ionic bonds occur between metals, losing electrons, and nonmetals, gaining electrons. Web compounds between metals and nonmetals are predominantly ionic because there is a large difference in electronegativity between most metals and most. Ions with opposite charges will attract one another creating an ionic bond..
Ionic, Covalent, and Metallic Bonds Differences and Similarities
Web nonmetals lose electrons to gain negative charge and form anions, whereas metals lose electrons to gain positive charge and form cations. Ionic bonds form only between metals and nonmetals. Ionic bonds are chemical bonds formed by the transfer of electrons from a metal to a nonmetal. Are oxidized) when they undergo chemical reactions they. Ionic bonds are the strongest.
Ionic Bonds Are The Strongest Type Of Bond In The World And.
Web ionic bonds occur between metals, losing electrons, and nonmetals, gaining electrons. Ions with opposite charges will attract one another creating an ionic bond. Web the interaction of metals and halogens results in the creation of ionic bonds. Ionic bonds form only between metals and nonmetals.
Are Oxidized) When They Undergo Chemical Reactions They.
An ionic bond is a type of. Web when ionic bonds form, a metal donates one or more electrons, due to having a low electronegativity, to form a positive ion or cation. That’s because metals want to give up electrons, and nonmetals want to gain electrons. That’s because metals “want” to give up electrons, and nonmetals “want” to gain electrons.
Web Ionic Bonds Form Only Between Metals And Nonmetals.
Web answer (1 of 6): Anions are ions that have negative. Web compounds between metals and nonmetals are predominantly ionic because there is a large difference in electronegativity between most metals and most. Web as all ionic compounds are polar they form salts.
Web Ionic Bonds Form Only Between Metals And Nonmetals.
Salts(ionic compounds) are generally crystalline in nature and hence contain water of crystallization. That's because metals “want” to give up electrons, and nonmetals “want” to gain electrons. Web part of chemistry (single science) chemical patterns revise test 1 2 3 4 5 6 7 forming ionic compounds metalatoms have only a few electrons in their outer shell whereas non. Ionic bonds are chemical bonds formed by the transfer of electrons from a metal to a nonmetal.



:max_bytes(150000):strip_icc()/ionic-bond-58fd4ea73df78ca1590682ad.jpg)